Safety and immunogenicity of an HIV-1 prefusion-stabilized envelope trimer (Trimer 4571) vaccine in healthy adults: A first-in-human open-label, randomized, dose-escalation, phase 1 clinical trial
- PMID: 35783486
- PMCID: PMC9249552
- DOI: 10.1016/j.eclinm.2022.101477
Safety and immunogenicity of an HIV-1 prefusion-stabilized envelope trimer (Trimer 4571) vaccine in healthy adults: A first-in-human open-label, randomized, dose-escalation, phase 1 clinical trial
Abstract
Background: Advances in therapeutic drugs have increased life-expectancies for HIV-infected individuals, but the need for an effective vaccine remains. We assessed safety and immunogenicity of HIV-1 vaccine, Trimer 4571 (BG505 DS-SOSIP.664) adjuvanted with aluminum hydroxide (alum), in HIV-negative adults.
Methods: We conducted a phase I, randomized, open-label, dose-escalation trial at the National Institutes of Health Clinical Center in Bethesda, MD, USA. Eligible participants were HIV-negative, healthy adults between 18-50 years. Participants were randomized 1:1 to receive Trimer 4571 adjuvanted with 500 mcg alum by either the subcutaneous (SC) or intramuscular (IM) route at weeks 0, 8, and 20 in escalating doses of 100 mcg or 500 mcg. The primary objectives were to evaluate the safety and tolerability of Trimer 4571 with a secondary objective of evaluating vaccine-induced antibody responses. The primary and safety endpoints were evaluated in all participants who received at least one dose of Trimer 4571. Trial results were summarized using descriptive statistics. This trial is registered at ClinicalTrials.gov, NCT03783130.
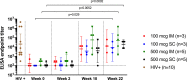
Findings: Between March 7 and September 11, 2019, 16 HIV-negative participants were enrolled, including six (38%) males and ten (62%) females. All participants received three doses of Trimer 4571. Solicited reactogenicity was mild to moderate in severity, with one isolated instance of severe injection site redness (6%) following a third 500 mcg SC administration. The most commonly reported solicited symptoms included mild injection site tenderness in 14 (88%) and mild myalgia in six (38%) participants. The most frequent unsolicited adverse event attributed to vaccination was mild injection site pruritus in six (38%) participants. Vaccine-induced seropositivity occurred in seven (44%) participants and resolved in all but one (6%). No serious adverse events occurred. Trimer 4571-specific binding antibodies were detected in all groups two weeks after regimen completion, primarily focused on the glycan-free trimer base. Neutralizing antibody activity was limited to the 500 mcg dose groups.
Interpretation: Trimer 4571 was safe, well tolerated, and immunogenic in this first-in-human trial. While this phase 1 trial is limited in size, our results inform and support further evaluation of prefusion-stabilized HIV-1 envelope trimers as a component of vaccine design strategies to generate broadly neutralizing antibodies against HIV-1.
Funding: Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Keywords: BG505 DS-SOSIP.664; HIV-1; NIH; Phase 1 clinical trial; Trimer 4571; Vaccine.
© 2022 The Authors.
Conflict of interest statement
CC, HG, JRM, and PDK are listed on patent applications involving Trimer 4571. All other authors declare no competing interests.
Figures




References
-
- Fauci AS, Lane HC. Four decades of HIV/AIDS - much accomplished, much to do. N Engl J Med. 2020;383(1):1–4. - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. - PubMed
-
- Kwong PD, Mascola JR. HIV-1 vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity. 2018;48(5):855–871. - PubMed
-
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191(5):654–665. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

