The Efficiency of Convalescent Plasma Therapy in the Management of Critically Ill Patients Infected With COVID-19: A Matched Cohort Study
- PMID: 35783610
- PMCID: PMC9243335
- DOI: 10.3389/fmed.2022.822821
The Efficiency of Convalescent Plasma Therapy in the Management of Critically Ill Patients Infected With COVID-19: A Matched Cohort Study
Abstract
Background: The convalescent plasma of patients who recover from coronavirus disease 2019 (COVID-19) contains high titers of neutralizing antibodies, which has potential effects on the viral shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and improving the prognosis of patients with COVID-19. The goal of this study was to clarify the effects of convalescent plasma therapy on the 60-day mortality and negative conversion rate of SARS-CoV-2 during the hospitalization of patients with severe and life-threatening COVID-19 infection.
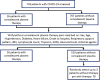
Methods: This was a retrospective, case-matched cohort study that involved patients with severe COVID-19 infections. The patients who received convalescent plasma therapy were matched by age, sex, diabetes, hypertension, heart failure, the onset of symptoms to hospital admission, respiratory support pattern, lymphocyte count, troponin, Sequential organ failure assessment (SOFA), glucocorticoid, and antiviral agents to no more than three patients with COVID-19 who did not receive convalescent plasma therapy. A Cox regression model and competing risk analysis were used to evaluate the effects of convalescent plasma therapy on these patients.
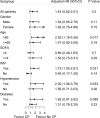
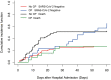
Results: Twenty-six patients were in the convalescent plasma therapy group, and 78 patients were in the control group. Demographic characteristics were similar in both groups, except for the SOFA score. Convalescent plasma therapy did not improve 60-day mortality [hazard ratio (HR) 1.44, 95% CI 0.82-2.51, p = 0.20], but the SARS-CoV-2 negative conversion rate for 60 days after admission was higher in the convalescent plasma group (26.9 vs. 65.4%, p = 0.002) than in the control. Then, a competing risk analysis was performed, which considered events of interest (the negative conversion rate of SARS-CoV-2) and competing events (death) in the same model. Convalescent plasma therapy improved events of interest (p = 0.0002).
Conclusion: Convalescent plasma therapy could improve the SARS-CoV-2 negative conversion rate but could not improve 60-day mortality in patients with severe and life-threatening COVID-19 infection.
Clinical trial number: The study was registered at ClinicalTrials.gov (NCT04616976).
Keywords: COVID-19; convalescent plasma therapy; mechanical ventilation; mortality; viral shedding.
Copyright © 2022 Pan, Chen, Xie, Huang, Yang, Du and Qiu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma.JAMA. 2020 Apr 28;323(16):1582-1589. doi: 10.1001/jama.2020.4783. JAMA. 2020. PMID: 32219428 Free PMC article.
-
Randomized clinical trial to evaluate safety and efficacy of convalescent plasma use among hospitalized patients with COVID-19 (PERUCONPLASMA): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 May 17;22(1):342. doi: 10.1186/s13063-021-05189-6. Trials. 2021. PMID: 34001174 Free PMC article.
-
Reconvalescent plasma/camostat mesylate in early SARS-CoV-2 Q-PCR positive high-risk individuals (RES-Q-HR): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 May 17;22(1):343. doi: 10.1186/s13063-021-05181-0. Trials. 2021. PMID: 34001215 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. Updated.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2020 Oct 12;10:CD013600. doi: 10.1002/14651858.CD013600.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 May 20;5:CD013600. doi: 10.1002/14651858.CD013600.pub4. PMID: 33044747 Updated.
Cited by
-
The effect of convalescent plasma therapy on the rate of nucleic acid negative conversion in patients with persistent COVID-19 test positivity.Front Pharmacol. 2024 Aug 1;15:1421516. doi: 10.3389/fphar.2024.1421516. eCollection 2024. Front Pharmacol. 2024. PMID: 39148549 Free PMC article.
-
Effectiveness of COVID-19 Convalescent Plasma (CCP) During the Pandemic Era: A Literature Review.J Blood Med. 2023 Feb 22;14:159-187. doi: 10.2147/JBM.S397722. eCollection 2023. J Blood Med. 2023. PMID: 36855559 Free PMC article. Review.
-
Rates Among Hospitalized Patients With COVID-19 Treated With Convalescent Plasma: A Systematic Review and Meta-Analysis.Mayo Clin Proc Innov Qual Outcomes. 2023 Oct 10;7(5):499-513. doi: 10.1016/j.mayocpiqo.2023.09.001. eCollection 2023 Oct. Mayo Clin Proc Innov Qual Outcomes. 2023. PMID: 37859995 Free PMC article.
References
-
- WHO . WHO Coronavirus Disease (COVID-19) Dashboard (2020). Available online at: https://covid19.who.int/ (accessed May 25, 2020).
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

