Gene Edited T Cell Therapies for Inborn Errors of Immunity
- PMID: 35783679
- PMCID: PMC9244397
- DOI: 10.3389/fgeed.2022.899294
Gene Edited T Cell Therapies for Inborn Errors of Immunity
Abstract
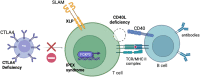
Inborn errors of immunity (IEIs) are a heterogeneous group of inherited disorders of the immune system. Many IEIs have a severe clinical phenotype that results in progressive morbidity and premature mortality. Over 450 IEIs have been described and the incidence of all IEIs is 1/1,000-10,000 people. Current treatment options are unsatisfactory for many IEIs. Allogeneic haematopoietic stem cell transplantation (alloHSCT) is curative but requires the availability of a suitable donor and carries a risk of graft failure, graft rejection and graft-versus-host disease (GvHD). Autologous gene therapy (GT) offers a cure whilst abrogating the immunological complications of alloHSCT. Gene editing (GE) technologies allow the precise modification of an organisms' DNA at a base-pair level. In the context of genetic disease, this enables correction of genetic defects whilst preserving the endogenous gene control machinery. Gene editing technologies have the potential to transform the treatment landscape of IEIs. In contrast to gene addition techniques, gene editing using the CRISPR system repairs or replaces the mutation in the DNA. Many IEIs are limited to the lymphoid compartment and may be amenable to T cell correction alone (rather than haematopoietic stem cells). T cell Gene editing has the advantages of higher editing efficiencies, reduced risk of deleterious off-target edits in terminally differentiated cells and less toxic conditioning required for engraftment of lymphocytes. Although most T cells lack the self-renewing property of HSCs, a population of T cells, the T stem cell memory compartment has long-term multipotent and self-renewal capacity. Gene edited T cell therapies for IEIs are currently in development and may offer a less-toxic curative therapy to patients affected by certain IEIs. In this review, we discuss the history of T cell gene therapy, developments in T cell gene editing cellular therapies before detailing exciting pre-clinical studies that demonstrate gene editing T cell therapies as a proof-of-concept for several IEIs.
Keywords: cellular therapeutics; gene editing; gene therapeutics; inborn error of immunity (IEI); primary immumunodeficiencies; t cell.
Copyright © 2022 Fox, Houghton and Booth.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

