Pericellular heparan sulfate proteoglycans: Role in regulating the biosynthetic response of nucleus pulposus cells to osmotic loading
- PMID: 35783912
- PMCID: PMC9238280
- DOI: 10.1002/jsp2.1209
Pericellular heparan sulfate proteoglycans: Role in regulating the biosynthetic response of nucleus pulposus cells to osmotic loading
Abstract
Background: Daily physiologic loading causes fluctuations in hydration of the intervertebral disc (IVD); thus, the embedded cells experience cyclic alterations to their osmotic environment. These osmotic fluctuations have been described as a mechanism linking mechanics and biology, and have previously been shown to promote biosynthesis in chondrocytes. However, this phenomenon has yet to be fully interrogated in the IVD. Additionally, the specialized extracellular matrix surrounding the cells, the pericellular matrix (PCM), transduces the biophysical signals that cells ultimately experience. While it is known that the PCM is altered in disc degeneration, whether it disrupts normal osmotic mechanotransduction has yet to be determined. Thus, our objectives were to assess: (1) whether dynamic osmotic conditions stimulate biosynthesis in nucleus pulposus cells, and (2) whether pericellular heparan sulfate proteoglycans (HSPGs) modulate the biosynthetic response to osmotic loading.
Methods: Bovine nucleus pulposus cells isolated with retained PCM were encapsulated in 1.5% alginate beads and treated with or without heparinase III, an enzyme that degrades the pericellular HSPGs. Beads were subjected to 1 h of daily iso-osmotic, hyper-osmotic, or hypo-osmotic loading for 1, 2, or 4 weeks. At each timepoint the total amount of extracellular and pericellular sGAG/DNA were quantified. Additionally, whether osmotic loading triggered alterations to HSPG sulfation was assessed via immunohistochemistry for the heparan sulfate 6-O-sulfertransferase 1 (HS6ST1) enzyme.
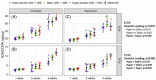
Results: Osmotic loading significantly influenced sGAG/DNA accumulation with a hyper-osmotic change promoting the greatest sGAG/DNA accumulation in the pericellular region compared with iso-osmotic conditions. Heparanase-III treatment significantly reduced extracellular sGAG/DNA but pericellular sGAG was not affected. HS6ST1 expression was not affected by osmotic loading.
Conclusion: Results suggest that hyper-osmotic loading promotes matrix synthesis and that modifications to HSPGs directly influence the metabolic responses of cells to osmotic fluctuations. Collectively, results suggest degeneration-associated modifications to pericellular HSPGs may contribute to the altered mechanobiology observed in disease.
Keywords: heparan sulfate proteoglycan; intervertebral disc; mechanotransduction; nucleus pulposus; osmotic; pericellular matrix.
© 2022 The Authors. JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Notochordal cell produce and assemble extracellular matrix in a distinct manner, which may be responsible for the maintenance of healthy nucleus pulposus.Spine (Phila Pa 1976). 2006 Apr 15;31(8):873-82; discussion 883. doi: 10.1097/01.brs.0000209302.00820.fd. Spine (Phila Pa 1976). 2006. PMID: 16622374
-
Matrix protein gene expression in intervertebral disc cells subjected to altered osmolarity.Biochem Biophys Res Commun. 2002 May 10;293(3):932-8. doi: 10.1016/S0006-291X(02)00314-5. Biochem Biophys Res Commun. 2002. PMID: 12051748
-
Influence of diurnal hyperosmotic loading on the metabolism and matrix gene expression of a whole-organ intervertebral disc model.J Orthop Res. 2006 Oct;24(10):1957-66. doi: 10.1002/jor.20243. J Orthop Res. 2006. PMID: 16917902
-
Mechanobiology of the intervertebral disc and relevance to disc degeneration.J Bone Joint Surg Am. 2006 Apr;88 Suppl 2:52-7. doi: 10.2106/JBJS.F.00001. J Bone Joint Surg Am. 2006. PMID: 16595444 Review.
-
Pentosan Polysulfate, a Semisynthetic Heparinoid Disease-Modifying Osteoarthritic Drug with Roles in Intervertebral Disc Repair Biology Emulating the Stem Cell Instructive and Tissue Reparative Properties of Heparan Sulfate.Stem Cells Dev. 2022 Aug;31(15-16):406-430. doi: 10.1089/scd.2022.0007. Epub 2022 Mar 14. Stem Cells Dev. 2022. PMID: 35102748 Review.
Cited by
-
Diverse and multifunctional roles for perlecan (HSPG2) in repair of the intervertebral disc.JOR Spine. 2024 Jul 29;7(3):e1362. doi: 10.1002/jsp2.1362. eCollection 2024 Sep. JOR Spine. 2024. PMID: 39081381 Free PMC article. Review.
-
Glycosaminoglycans, Instructive Biomolecules That Regulate Cellular Activity and Synaptic Neuronal Control of Specific Tissue Functional Properties.Int J Mol Sci. 2025 Mar 12;26(6):2554. doi: 10.3390/ijms26062554. Int J Mol Sci. 2025. PMID: 40141196 Free PMC article. Review.
-
Spatial and Diurnal Variations in Sodium Content Within Intervertebral Disc Tissue.JOR Spine. 2025 Aug 5;8(3):e70079. doi: 10.1002/jsp2.70079. eCollection 2025 Sep. JOR Spine. 2025. PMID: 40766839 Free PMC article.
-
Impaired instructive and protective barrier functions of the endothelial cell glycocalyx pericellular matrix is impacted in COVID-19 disease.J Cell Mol Med. 2024 Aug;28(16):e70033. doi: 10.1111/jcmm.70033. J Cell Mol Med. 2024. PMID: 39180511 Free PMC article. Review.
References
-
- Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245‐258. - PubMed
-
- Buschmann MD, Grodzinsky AJ. A molecular model of proteoglycan‐associated electrostatic forces in cartilage mechanics. J Biomech Eng. 1995;117:179‐192. - PubMed
-
- Urban JP, Maroudas A, Bayliss MT, Dillon J. Swelling pressures of proteoglycans at the concentrations found in cartilaginous tissues. Biorheology. 1979;16:447‐464. - PubMed
-
- Ishihara H, Warensjo K, Roberts S, Urban JP. Proteoglycan synthesis in the intervertebral disk nucleus: the role of extracellular osmolality. Am J Physiol. 1997;272:C1499‐C1506. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

