IL-1Ra deficiency accelerates intervertebral disc degeneration in C57BL6J mice
- PMID: 35783913
- PMCID: PMC9238285
- DOI: 10.1002/jsp2.1201
IL-1Ra deficiency accelerates intervertebral disc degeneration in C57BL6J mice
Abstract
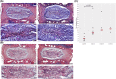
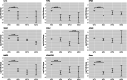
The expression of Interleukin-1ß (IL-1ß) and its antagonist and Interleukin-1 receptor antagonist (IL-1Ra) are correlated with greater human intervertebral disc (IVD) degeneration, suggesting that elevated IL-1β activity promotes disc degeneration. Many in vitro studies support such a mechanistic relationship, whereas few in vivo investigations have been reported. The present study tests the effect of increased IL-1β activity on intervertebral disc in mice with an IL-1Ra gene deletion. IL-1Ra-/- mice and wild-type (WT) C57Bl6J mice were examined at 3 and 12 months of age. Caudal IVD segments were evaluated for disc degeneration by histopathology, functional testing, and inflammatory gene expression relevant to IL-1β pathways. To test differences in injury response, pinprick annular puncture was performed on IL-1Ra-/- and WT mice and evaluated similarly. IL-1Ra-/- IVDs had significantly worse histopathology at 3 months compared to WT controls, but not at 12 months. IL-1Ra-/- IVDs exhibited significantly more viscous mechanical properties than WT IVDs. qPCR revealed downregulation of inflammatory genes at 3 and 12 months in IL-1Ra-/- IVDs, with concomitant downregulation of anabolic and catabolic genes. Annular puncture yielded no appreciable differences between 2-week and 6-week post-injured WT and IL1-Ra-/- IVDs in histopathology or biomechanics, but inflammatory gene expression was sharply downregulated in IL-1Ra-/- mice at 2 weeks, returning by 6 weeks post injury. In the present study, IL-1Ra deletion resulted in increased IVD histopathology, inferior biomechanics, and transiently decreased pro-inflammatory cytokine gene expression. The histopathology of IL-1Ra-/- IVDs on a C57BL/6J background is less severe than a previous report of IL1Ra-/- on a BALB/c background, yet both strains exhibit IVD degeneration, reinforcing a mechanistic role of IL-1β signaling in IVD pathobiology. Despite a pro-inflammatory environment, the annular puncture was no worse in IL-1Ra-/- mice, suggesting that response to injury involves pathways other than inflammation. Overall, this study supports the hypothesis that IL-1β-driven inflammation is important in IVD degeneration.
Keywords: IL‐1ra deficiency; intervertebral disc; mouse strain.
© 2022 The Authors. JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Figures





Similar articles
-
HMGB1 Mediates Macrophage Recruitment and Regional Intervertebral Disc Properties Following Injury.FASEB J. 2025 Jun 30;39(12):e70711. doi: 10.1096/fj.202402658R. FASEB J. 2025. PMID: 40536102
-
Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study.Arthritis Res Ther. 2007;9(4):R83. doi: 10.1186/ar2282. Arthritis Res Ther. 2007. PMID: 17760968 Free PMC article.
-
NF-κB inhibitor, NEMO-binding domain peptide attenuates intervertebral disc degeneration.Spine J. 2020 Sep;20(9):1480-1491. doi: 10.1016/j.spinee.2020.04.025. Epub 2020 May 12. Spine J. 2020. PMID: 32413485 Free PMC article.
-
Deletion of Opg Leads to Increased Neovascularization and Expression of Inflammatory Cytokines in the Lumbar Intervertebral Disc of Mice.Spine (Phila Pa 1976). 2017 Jan 1;42(1):E8-E14. doi: 10.1097/BRS.0000000000001701. Spine (Phila Pa 1976). 2017. PMID: 27196016
-
Role of neurogenic inflammation in intervertebral disc degeneration.World J Orthop. 2025 Jan 18;16(1):102120. doi: 10.5312/wjo.v16.i1.102120. eCollection 2025 Jan 18. World J Orthop. 2025. PMID: 39850033 Free PMC article. Review.
Cited by
-
PCRX-201, a novel IL-1Ra gene therapy treatment approach for low back pain resulting from intervertebral disc degeneration.Gene Ther. 2025 Mar;32(2):93-105. doi: 10.1038/s41434-024-00504-7. Epub 2024 Nov 21. Gene Ther. 2025. PMID: 39572769 Free PMC article.
-
Regulatory Effect of Inflammatory Mediators in Intervertebral Disc Degeneration.Mediators Inflamm. 2023 Apr 17;2023:6210885. doi: 10.1155/2023/6210885. eCollection 2023. Mediators Inflamm. 2023. PMID: 37101594 Free PMC article. Review.
-
Nuclear factor κB overactivation in the intervertebral disc leads to macrophage recruitment and severe disc degeneration.Sci Adv. 2024 Jun 7;10(23):eadj3194. doi: 10.1126/sciadv.adj3194. Epub 2024 Jun 7. Sci Adv. 2024. PMID: 38848366 Free PMC article.
-
Inactivation of Tnf-α/Tnfr signaling attenuates progression of intervertebral disc degeneration in mice.JOR Spine. 2024 Oct 8;7(4):e70006. doi: 10.1002/jsp2.70006. eCollection 2024 Dec. JOR Spine. 2024. PMID: 39391171 Free PMC article.
References
-
- Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age‐related changes in lumbar intervertebral discs. Spine. 2002;27:2631‐2644. - PubMed
-
- Schmorl G, Junghanns H. The Human Spine in Health and Disease. Grune & Stratton; 1959.
-
- Franceschi C. Inflamm‐aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244‐254. - PubMed
LinkOut - more resources
Full Text Sources

