Structural and Biophysical Characterization of Purified Recombinant Arabidopsis thaliana's Alternative Oxidase 1A (rAtAOX1A): Interaction With Inhibitor(s) and Activator
- PMID: 35783971
- PMCID: PMC9243770
- DOI: 10.3389/fpls.2022.871208
Structural and Biophysical Characterization of Purified Recombinant Arabidopsis thaliana's Alternative Oxidase 1A (rAtAOX1A): Interaction With Inhibitor(s) and Activator
Abstract
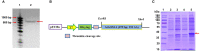
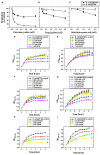
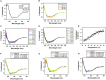
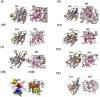
In higher plants, alternative oxidase (AOX) participates in a cyanide resistant and non-proton motive electron transport pathway of mitochondria, diverging from the ubiquinone pool. The physiological significance of AOX in biotic/abiotic stress tolerance is well-documented. However, its structural and biophysical properties are poorly understood as its crystal structure is not yet revealed in plants. Also, most of the AOX purification processes resulted in a low yield/inactive/unstable form of native AOX protein. The present study aims to characterize the purified rAtAOX1A protein and its interaction with inhibitors, such as salicylhydroxamic acid (SHAM) and n-propyl gallate (n-PG), as well as pyruvate (activator), using biophysical/in silico studies. The rAtAOX1A expressed in E. coli BL21(DE3) cells was functionally characterized by monitoring the respiratory and growth sensitivity of E. coli/pAtAOX1A and E. coli/pET28a to classical mitochondrial electron transport chain (mETC) inhibitors. The rAtAOX1A, which is purified through affinity chromatography and confirmed by western blotting and MALDI-TOF-TOF studies, showed an oxygen uptake activity of 3.86 μmol min-1 mg-1 protein, which is acceptable in non-thermogenic plants. Circular dichroism (CD) studies of purified rAtAOX1A revealed that >50% of the protein content was α-helical and retained its helical absorbance signal (ellipticity) at a wide range of temperature and pH conditions. Further, interaction with SHAM, n-PG, or pyruvate caused significant changes in its secondary structural elements while retaining its ellipticity. Surface plasmon resonance (SPR) studies revealed that both SHAM and n-PG bind reversibly to rAtAOX1A, while docking studies revealed that they bind to the same hydrophobic groove (Met191, Val192, Met195, Leu196, Phe251, and Phe255), to which Duroquinone (DQ) bind in the AtAOX1A. In contrast, pyruvate binds to a pocket consisting of Cys II (Arg174, Tyr175, Gly176, Cys177, Val232, Ala233, Asn294, and Leu313). Further, the mutational docking studies suggest that (i) the Met195 and Phe255 of AtAOX1A are the potential candidates to bind the inhibitor. Hence, this binding pocket could be a 'potential gateway' for the oxidation-reduction process in AtAOX1A, and (ii) Arg174, Gly176, and Cys177 play an important role in binding to the organic acids like pyruvate.
Keywords: AOX structure; Arabidopsis thaliana; alternative oxidase; circular dichroism; mutational docking; recombinant protein; respiratory inhibitors; surface plasmon resonance.
Copyright © 2022 Sankar, Saharay, Santhosh, Vishwakarma and Padmasree.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Anraku Y., Gennis R. B. (1987). The aerobic respiratory chain of Escherichia coli. Trends Biochem. Sci. 12, 262–266. 10.1016/0968-0004(87)90131-9 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

