Salvianolic Acid Modulates Physiological Responses and Stress-Related Genes That Affect Osmotic Stress Tolerance in Glycine max and Zea mays
- PMID: 35783988
- PMCID: PMC9240475
- DOI: 10.3389/fpls.2022.904037
Salvianolic Acid Modulates Physiological Responses and Stress-Related Genes That Affect Osmotic Stress Tolerance in Glycine max and Zea mays
Abstract
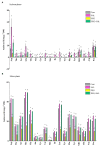
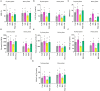
Drought is a serious threat worldwide to soybean and maize production. This study was conducted to discern the impact of salvianolic acid treatment on osmotic-stressed soybean (Glycine max L.) and maize (Zea mays L.) seedlings from the perspective of physiochemical and molecular reactions. Examination of varied salvianolic acid concentrations (0, 0.1, 1, 5, 10, and 25 μM) on soybean and maize seedling growth confirmed that the 0.1 and 1 μM concentrations, respectively, showed an improvement in agronomic traits. Likewise, the investigation ascertained how salvianolic acid application could retrieve osmotic-stressed plants. Soybean and maize seedlings were irrigated with water or 25% PEG for 8 days. The results indicated that salvianolic acid application promoted the survival of the 39-day-old osmotic-stressed soybean and maize plants. The salvianolic acid-treated plants retained high photosynthetic pigments, protein, amino acid, fatty acid, sugar, and antioxidant contents, and demonstrated low hydrogen peroxide and lipid contents under osmotic stress conditions. Gene transcription pattern certified that salvianolic acid application led to an increased expression of GmGOGAT, GmUBC2, ZmpsbA, ZmNAGK, ZmVPP1, and ZmSCE1d genes, and a diminished expression of GmMIPS2, GmSOG1, GmACS, GmCKX, ZmPIS, and ZmNAC48 genes. Together, our results indicate the utility of salvianolic acid to enhance the osmotic endurance of soybean and maize plants.
Keywords: amino acid; antioxidant enzymes; fatty acid; hydrogen peroxide; lipid metabolism; protein; sugar.
Copyright © 2022 Kazerooni, Al-Sadi, Rashid, Kim, Kang and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Ampelopsin Confers Endurance and Rehabilitation Mechanisms in Glycine max cv. Sowonkong under Multiple Abiotic Stresses.Int J Mol Sci. 2021 Oct 10;22(20):10943. doi: 10.3390/ijms222010943. Int J Mol Sci. 2021. PMID: 34681604 Free PMC article.
-
Acetic acid positively modulates proline metabolism for mitigating PEG-mediated drought stress in Maize and Arabidopsis.Front Plant Sci. 2023 Jul 19;14:1167238. doi: 10.3389/fpls.2023.1167238. eCollection 2023. Front Plant Sci. 2023. PMID: 37538054 Free PMC article.
-
Seed Priming Modulates Physiological and Agronomic Attributes of Maize (Zea mays L.) under Induced Polyethylene Glycol Osmotic Stress.ACS Omega. 2023 Jun 15;8(25):22788-22808. doi: 10.1021/acsomega.3c01715. eCollection 2023 Jun 27. ACS Omega. 2023. PMID: 37396236 Free PMC article.
-
Physio-morphological traits and osmoregulation strategies of hybrid maize (Zea mays) at the seedling stage in response to water-deficit stress.Protoplasma. 2022 Jul;259(4):869-883. doi: 10.1007/s00709-021-01707-0. Epub 2021 Sep 28. Protoplasma. 2022. PMID: 34581924
-
Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine max L.) seedlings.J Sci Food Agric. 2021 Mar 30;101(5):2027-2041. doi: 10.1002/jsfa.10822. Epub 2020 Oct 6. J Sci Food Agric. 2021. PMID: 32949013
Cited by
-
To Be or Not to Be? Are Reactive Oxygen Species, Antioxidants, and Stress Signalling Universal Determinants of Life or Death?Cells. 2022 Dec 17;11(24):4105. doi: 10.3390/cells11244105. Cells. 2022. PMID: 36552869 Free PMC article. Review.
-
Exogenous SA Applications Alleviate Salinity Stress via Physiological and Biochemical changes in St John's Wort Plants.Plants (Basel). 2023 Jan 9;12(2):310. doi: 10.3390/plants12020310. Plants (Basel). 2023. PMID: 36679023 Free PMC article.
References
-
- Abdoulaye A. O., Lu H., Zhu Y., Hamoud Y. A., Sheteiwy M. (2019). The global trend of the net irrigation water requirement of maize from 1960 to 2050. Climate 7, 124. 10.3390/cli7100124 - DOI
-
- Agarwal S., Pandey V. (2004). Antioxidant enzyme responses to NaCl stress in cassia angustifolia. Biologia Plantarum 48, 555–560. 10.1023/B:BIOP.0000047152.07878.e7 - DOI
-
- Anjaneyulu E., Reddy P. S., Sunita M. S., Kishor P. B. K., Meriga B. (2014). Salt tolerance and activity of antioxidative enzymes of transgenic finger millet overexpressing a vacuolar H+-pyrophosphatase gene (SbVPPase) from Sorghum bicolor. J. Plant Physiol. 171, 789–798. 10.1016/j.jplph.2014.02.001 - DOI - PubMed
LinkOut - more resources
Full Text Sources

