Cell-Free Blood Cell Secretome (BCS) Counteracts Skin Aging: Multi-Center Prospective Regenerative Aesthetic Medicine Study Using Exokine®
- PMID: 35784268
- PMCID: PMC9248816
- DOI: 10.2147/CCID.S357810
Cell-Free Blood Cell Secretome (BCS) Counteracts Skin Aging: Multi-Center Prospective Regenerative Aesthetic Medicine Study Using Exokine®
Abstract
Background: The "Inflammation Theory of Ageing" identifies pro-inflammatory cytokines and oxidative damage as one cause of cellular and mitochondrial deterioration and aging. Cell-free blood cell secretome (BCS) also known as autologous conditioned serum (ACS) has shown anti-inflammatory and regenerative mode of action in musculoskeletal disorders and radicular compression.
Aim: To confirm that BCS can improve signs of skin aging from a previous study in a multi-center setting.
Methods: Prospective, one-armed, multi-center interventional therapeutic study. Ninety-five women with skin firmness loss were treated with four intra-dermal injection sessions in both cheeks at 0, 2, 4 and 6 weeks. BCS was processed with Exokine® medical device according to manufacturer's instructions. Primary endpoints were cutometric R0 and R3 at 12 and 24 weeks. GAIS, FACE-QTM, Patient Attractivity Self-Assessment and safety were evaluated.
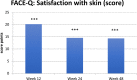
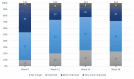
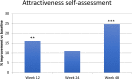
Results: Mean skin firmness (R0) improved significantly from baseline 0.40 mm to 0.38 mm at week 12 and to 0.36 mm at week 24. Mean skin tiring (R3) improved significantly from baseline 0.45 mm to 0.42 mm at week 12 and to 0.40 at week 24. FACE-QTM "Satisfaction with Skin" significantly improved from baseline to weeks 12, 24 and 48. So did "Satisfaction with Facial Appearance" and "Psychological and Social Function". "Satisfaction with Decision" and "Satisfaction with Outcome" were stable at week 24 and 48. At week 48 patients assessed their age 1.68 years younger vs Baseline. FACE-QTM aging appraisal improves from Baseline 52.94 to 65.23 at week 48. GAIS, by both physicians and patients, confirm improvement of skin.
Conclusion: For up to 48 weeks four intra-dermal injections with cell-free BCS increase facial skin firmness and resilience to tiring and patients' satisfaction with their facial appearance and skin. Patients perceive their face as younger. BCS has the ability to sustainably rejuvenate facial skin safely.
Study registration: Registration on German clinical trials register: DRKS00013014.
Keywords: ACS; BCS; autologous; autologous conditioned serum; cell-free blood cell secretome; regeneration; rejuvenation; skin quality.
© 2022 Kerscher et al.
Conflict of interest statement
Prof. Dr. Martina Kerscher reports personal fees and study support by providing study materials from Orthogen, during the conduct of the study. Dr Tanja Fischer reports grants from Orthogen, during the conduct of the study. Dr Tanju Kaptan was an Employee of Orthogen AG. Dr Glyn Hamed reports personal fees from Orthogen AG, during the writing of the manuscript and outside the submitted work. Dr Julio Reinecke is an employee of Orthogen, outside the submitted work; In addition, Dr Julio Reinecke shares a patent WO2017080669A1 pending to Orthogen AG. The other authors report no conflicts of interest in this work.
Figures




Similar articles
-
Cell-Free Blood Cell Secretome (BCS) Counteracts Skin Aging and Restores Firmness and Elasticity.J Drugs Dermatol. 2021 Jun 1;20(6):682-688. doi: 10.36849/JDD.2021.5018. J Drugs Dermatol. 2021. PMID: 34076388
-
Multifractional microablative laser combined with spacially modulated ablative (SMA) technology for facial skin rejuvenation.Lasers Surg Med. 2017 Jan;49(1):78-83. doi: 10.1002/lsm.22561. Epub 2016 Jul 18. Lasers Surg Med. 2017. PMID: 27426249
-
Injectable platelet-rich fibrin for facial rejuvenation: A prospective, single-center study.J Cosmet Dermatol. 2020 Dec;19(12):3213-3221. doi: 10.1111/jocd.13692. Epub 2020 Sep 23. J Cosmet Dermatol. 2020. PMID: 32852873
-
Effect of stem cell secretome in skin rejuvenation: a narrative review.Mol Biol Rep. 2023 Sep;50(9):7745-7758. doi: 10.1007/s11033-023-08622-y. Epub 2023 Jul 15. Mol Biol Rep. 2023. PMID: 37452901 Review.
-
Development of a core outcome set for clinical trials in facial aging: study protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey.Trials. 2017 Aug 1;18(1):359. doi: 10.1186/s13063-017-2104-3. Trials. 2017. PMID: 28764734 Free PMC article.
Cited by
-
Approaches in line with human physiology to prevent skin aging.Front Physiol. 2023 Oct 25;14:1279371. doi: 10.3389/fphys.2023.1279371. eCollection 2023. Front Physiol. 2023. PMID: 37954446 Free PMC article. Review.
-
Recent clinical trials with stem cells to slow or reverse normal aging processes.Front Aging. 2023 Apr 6;4:1148926. doi: 10.3389/fragi.2023.1148926. eCollection 2023. Front Aging. 2023. PMID: 37090485 Free PMC article. Review.
-
Potential Utilisation of Secretome from Ascorbic Acid-Supplemented Stem Cells in Combating Skin Aging: Systematic Review of A Novel Idea.Cell J. 2023 Sep 9;25(9):591-602. doi: 10.22074/cellj.2023.1995999.1253. Cell J. 2023. PMID: 37718762 Free PMC article.
-
Measuring the Impact of Surgical and Non-surgical Facial Cosmetic Interventions Using FACE-Q Aesthetic Module Scales: A Systematic Review and Meta-Analysis.Plast Surg (Oakv). 2024 Jan 23;33(3):403-418. doi: 10.1177/22925503231225480. eCollection 2025 Aug. Plast Surg (Oakv). 2024. PMID: 39553513 Free PMC article.
-
Regenerative Aesthetics: A Genuine Frontier or Just a Facet of Regenerative Medicine: A Systematic Review.Aesthetic Plast Surg. 2025 Jan;49(1):341-355. doi: 10.1007/s00266-024-04287-5. Epub 2024 Aug 28. Aesthetic Plast Surg. 2025. PMID: 39198280
References
Publication types
LinkOut - more resources
Full Text Sources

