Review: Sustainable Clinical Development of CAR-T Cells - Switching From Viral Transduction Towards CRISPR-Cas Gene Editing
- PMID: 35784280
- PMCID: PMC9248912
- DOI: 10.3389/fimmu.2022.865424
Review: Sustainable Clinical Development of CAR-T Cells - Switching From Viral Transduction Towards CRISPR-Cas Gene Editing
Abstract
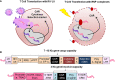
T cells modified for expression of Chimeric Antigen Receptors (CARs) were the first gene-modified cell products approved for use in cancer immunotherapy. CAR-T cells engineered with gammaretroviral or lentiviral vectors (RVs/LVs) targeting B-cell lymphomas and leukemias have shown excellent clinical efficacy and no malignant transformation due to insertional mutagenesis to date. Large-scale production of RVs/LVs under good-manufacturing practices for CAR-T cell manufacturing has soared in recent years. However, manufacturing of RVs/LVs remains complex and costly, representing a logistical bottleneck for CAR-T cell production. Emerging gene-editing technologies are fostering a new paradigm in synthetic biology for the engineering and production of CAR-T cells. Firstly, the generation of the modular reagents utilized for gene editing with the CRISPR-Cas systems can be scaled-up with high precision under good manufacturing practices, are interchangeable and can be more sustainable in the long-run through the lower material costs. Secondly, gene editing exploits the precise insertion of CARs into defined genomic loci and allows combinatorial gene knock-ins and knock-outs with exciting and dynamic perspectives for T cell engineering to improve their therapeutic efficacy. Thirdly, allogeneic edited CAR-effector cells could eventually become available as "off-the-shelf" products. This review addresses important points to consider regarding the status quo, pending needs and perspectives for the forthright evolution from the viral towards gene editing developments for CAR-T cells.
Keywords: CAR-T; CRISPR-Cas; GMP; gene editing; lentiviral; mouse models; retrovirus.
Copyright © 2022 Wagner, Koehl, Chmielewski, Scheid and Stripecke.
Conflict of interest statement
RS has filed a patent application for generation of CAR-T cells targeting lytic herpes infections and is a founding shareholder and scientific consultant of BioSyngen/Zelltechs Lpt Ltd. DW has filed multiple patent applications on CRISPR-Cas gene editing and adoptive T cell therapy. CS is consultant for Bristol Myers Squibb, Janssen and Novartis regarding CAR-T cell therapy and is participating in clinical CAR-T studies from Bristol Myers Squibb, Janssen, Novartis and Miltenyi Biotec and is cooperating with Miltenyi Biotec in the production of CAR-T cells. UK states that she is a consultant in immuno-oncology for AstraZeneca, Affimed, Glycostem, GammaDelta and Zelluna, and that she has collaborations with Novartis and Miltenyi Biotec regarding the production of CAR-T cells. MC is co-inventor in granted and filed patents describing CAR-T cells with additional functions to counteract the tumor microenvironment.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

