The 'Danse Macabre'-Neutrophils the Interactive Partner Affecting Oral Cancer Outcomes
- PMID: 35784290
- PMCID: PMC9243430
- DOI: 10.3389/fimmu.2022.894021
The 'Danse Macabre'-Neutrophils the Interactive Partner Affecting Oral Cancer Outcomes
Abstract
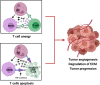
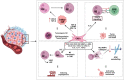
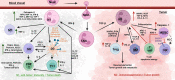
Over the past few decades, tremendous advances in the prevention, diagnosis, and treatment of cancer have taken place. However for head and neck cancers, including oral cancer, the overall survival rate is below 50% and they remain the seventh most common malignancy worldwide. These cancers are, commonly, aggressive, genetically complex, and difficult to treat and the delay, which often occurs between early recognition of symptoms and diagnosis, and the start of treatment of these cancers, is associated with poor prognosis. Cancer development and progression occurs in concert with alterations in the surrounding stroma, with the immune system being an essential element in this process. Despite neutrophils having major roles in the pathology of many diseases, they were thought to have little impact on cancer development and progression. Recent studies are now challenging this notion and placing neutrophils as central interactive players with other immune and tumor cells in affecting cancer pathology. This review focuses on how neutrophils and their sub-phenotypes, N1, N2, and myeloid-derived suppressor cells, both directly and indirectly affect the anti-tumor and pro-tumor immune responses. Emphasis is placed on what is currently known about the interaction of neutrophils with myeloid innate immune cells (such as dendritic cells and macrophages), innate lymphoid cells, natural killer cells, and fibroblasts to affect the tumor microenvironment and progression of oral cancer. A better understanding of this dialog will allow for improved therapeutics that concurrently target several components of the tumor microenvironment, increasing the possibility of constructive and positive outcomes for oral cancer patients. For this review, PubMed, Web of Science, and Google Scholar were searched for manuscripts using keywords and combinations thereof of "oral cancer, OSCC, neutrophils, TANs, MDSC, immune cells, head and neck cancer, and tumor microenvironment" with a focus on publications from 2018 to 2021.
Keywords: immune cells; innate immunity; interaction; myeloid cells; neutrophil; oral cancer; tumor microenvironment.
Copyright © 2022 Hadjigol, Shah and O’Brien-Simpson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The Tumor Microenvironment Innately Modulates Cancer Progression.Cancer Res. 2019 Sep 15;79(18):4557-4566. doi: 10.1158/0008-5472.CAN-18-3962. Epub 2019 Jul 26. Cancer Res. 2019. PMID: 31350295 Free PMC article. Review.
-
Mechanism of tumour microenvironment in the progression and development of oral cancer.Mol Biol Rep. 2021 Feb;48(2):1773-1786. doi: 10.1007/s11033-020-06054-6. Epub 2021 Jan 25. Mol Biol Rep. 2021. PMID: 33492572 Review.
-
Innate Immune Cells in the Esophageal Tumor Microenvironment.Front Immunol. 2021 Apr 28;12:654731. doi: 10.3389/fimmu.2021.654731. eCollection 2021. Front Immunol. 2021. PMID: 33995371 Free PMC article. Review.
-
Nanoparticles Targeting Innate Immune Cells in Tumor Microenvironment.Int J Mol Sci. 2021 Sep 16;22(18):10009. doi: 10.3390/ijms221810009. Int J Mol Sci. 2021. PMID: 34576180 Free PMC article. Review.
-
The role of innate immune cells in the colorectal cancer tumor microenvironment and advances in anti-tumor therapy research.Front Immunol. 2024 Jul 19;15:1407449. doi: 10.3389/fimmu.2024.1407449. eCollection 2024. Front Immunol. 2024. PMID: 39100676 Free PMC article. Review.
Cited by
-
Tumor microenvironment dynamics in oral cancer: unveiling the role of inflammatory cytokines in a syngeneic mouse model.Clin Exp Metastasis. 2024 Dec;41(6):891-908. doi: 10.1007/s10585-024-10306-1. Epub 2024 Aug 10. Clin Exp Metastasis. 2024. PMID: 39126553 Free PMC article.
-
Integrating single-cell RNA-seq and bulk RNA-seq to construct a neutrophil prognostic model for predicting prognosis and immune response in oral squamous cell carcinoma.Hum Genomics. 2024 Dec 26;18(1):140. doi: 10.1186/s40246-024-00712-7. Hum Genomics. 2024. PMID: 39726033 Free PMC article.
-
Multi-disciplinary approaches paving the way for clinically effective peptide vaccines for cancer.NPJ Vaccines. 2025 Apr 9;10(1):68. doi: 10.1038/s41541-025-01118-9. NPJ Vaccines. 2025. PMID: 40204832 Free PMC article. Review.
-
β-Lapachone promotes the recruitment and polarization of tumor-associated neutrophils (TANs) toward an antitumor (N1) phenotype in NQO1-positive cancers.Oncoimmunology. 2024 Jun 4;13(1):2363000. doi: 10.1080/2162402X.2024.2363000. eCollection 2024. Oncoimmunology. 2024. PMID: 38846085 Free PMC article.
-
Modulation of myeloid-derived suppressor cell functions by oral inflammatory diseases and important oral pathogens.Front Immunol. 2024 Mar 1;15:1349067. doi: 10.3389/fimmu.2024.1349067. eCollection 2024. Front Immunol. 2024. PMID: 38495880 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

