Tumor-Specific CD4+ T Cells Restrain Established Metastatic Melanoma by Developing Into Cytotoxic CD4- T Cells
- PMID: 35784297
- PMCID: PMC9243303
- DOI: 10.3389/fimmu.2022.875718
Tumor-Specific CD4+ T Cells Restrain Established Metastatic Melanoma by Developing Into Cytotoxic CD4- T Cells
Abstract
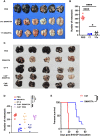
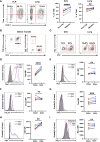
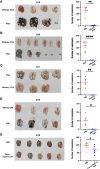
Cytotoxic CD8+ T cells are the main focus of efforts to understand anti-tumor immunity and immunotherapy. The adoptive transfer of tumor-reactive cytotoxic CD8+ T lymphocytes expanded and differentiated in vitro has long been considered the primary strategy in adaptive anti-tumor immunity, however, the majority of the transferred tumor antigen-specific CD8+ T cells differentiated into CD39+CD69+ exhausted progenies, limiting its effects in repressing tumor growth. Contrarily, less attention has been addressed to the role of CD4+ T cells during tumorigenesis. Using a mouse model of metastatic melanoma, we found that transferring tumor-specific CD4+ T cells into recipients induces substantial regression of the established metastatic tumors. Notably, in vitro activated CD4+ T cells developed into cytotoxic CD4- T cells in vivo and get exhausted gradually. The blockade of PD-L1 signaling resulted in an expansion of tumor specific CD4+ T cells, which could better control the established metastatic melanoma. Moreover, the tumor-specific memory CD4+ T cell can prevent mice from tumor metastasis, and the tumor-specific effector CD4+ T cells can also mitigate the established metastatic tumor. Overall, our findings suggest a novel mechanism of CD4+ T cells in curtailing tumor metastasis and confirm their therapeutic role in combination with PD-L1 blockade in cancer immunotherapy. Hence, a better understanding of cytotoxic CD4- T cell-mediated tumor regression could provide an alternative choice for patients exhibiting suboptimal or no response to CD8+ T cell-based immunotherapies.
Keywords: CD4+T cells; CD4-T cells; melanoma; metastasis; tumor specific.
Copyright © 2022 Liu, Wang, Lin, Wang, Wu, Guo, Wen, Ran, Yue, Su, Wu, Tang, Li, Hu, Xu, Ye and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
CD4+ T-cell epitope-based heterologous prime-boost vaccination potentiates anti-tumor immunity and PD-1/PD-L1 immunotherapy.J Immunother Cancer. 2022 May;10(5):e004022. doi: 10.1136/jitc-2021-004022. J Immunother Cancer. 2022. PMID: 35580929 Free PMC article.
-
PD-L1-Independent Mechanisms Control the Resistance of Melanoma to CD4+ T Cell Adoptive Immunotherapy.J Immunol. 2018 May 1;200(9):3304-3311. doi: 10.4049/jimmunol.1701617. Epub 2018 Mar 30. J Immunol. 2018. PMID: 29602773
-
Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts.J Exp Med. 2010 Mar 15;207(3):637-50. doi: 10.1084/jem.20091918. Epub 2010 Feb 15. J Exp Med. 2010. PMID: 20156971 Free PMC article.
-
Systemic CD4 Immunity as a Key Contributor to PD-L1/PD-1 Blockade Immunotherapy Efficacy.Front Immunol. 2020 Nov 30;11:586907. doi: 10.3389/fimmu.2020.586907. eCollection 2020. Front Immunol. 2020. PMID: 33329566 Free PMC article. Review.
-
Roles of CD4+ T cells as mediators of antitumor immunity.Front Immunol. 2022 Sep 9;13:972021. doi: 10.3389/fimmu.2022.972021. eCollection 2022. Front Immunol. 2022. PMID: 36159781 Free PMC article. Review.
Cited by
-
Tumor-specific memory CD8+ T cells are strictly resident in draining lymph nodes during tumorigenesis.Cell Mol Immunol. 2023 Apr;20(4):423-426. doi: 10.1038/s41423-023-00986-2. Epub 2023 Mar 1. Cell Mol Immunol. 2023. PMID: 36859455 Free PMC article. No abstract available.
-
Potential biomarkers: Identifying powerful tumor specific T cells in adoptive cellular therapy.Front Immunol. 2022 Nov 14;13:1003626. doi: 10.3389/fimmu.2022.1003626. eCollection 2022. Front Immunol. 2022. PMID: 36451828 Free PMC article. Review.
-
CD4+ T cells in antitumor immunity.Trends Cancer. 2024 Oct;10(10):969-985. doi: 10.1016/j.trecan.2024.07.009. Epub 2024 Sep 5. Trends Cancer. 2024. PMID: 39242276 Review.
-
CTLs heterogeneity and plasticity: implications for cancer immunotherapy.Mol Cancer. 2024 Mar 21;23(1):58. doi: 10.1186/s12943-024-01972-6. Mol Cancer. 2024. PMID: 38515134 Free PMC article. Review.
-
Two Modes of Th1 Polarization Induced by Dendritic-Cell-Priming Adjuvant in Vaccination.Cells. 2023 May 29;12(11):1504. doi: 10.3390/cells12111504. Cells. 2023. PMID: 37296625 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

