T-Follicular-Like CD8+ T Cell Responses in Chronic HIV Infection Are Associated With Virus Control and Antibody Isotype Switching to IgG
- PMID: 35784304
- PMCID: PMC9241491
- DOI: 10.3389/fimmu.2022.928039
T-Follicular-Like CD8+ T Cell Responses in Chronic HIV Infection Are Associated With Virus Control and Antibody Isotype Switching to IgG
Abstract
T cell responses are considered critical for the in vivo control of HIV, but the contribution of different T cell subsets to this control remains unclear. Using a boosted flow cytometric approach that is able to differentiate CD4+ and CD8+ T cell Th1/Tc1, Th2/Tc2, Th17/Tc17, Treg and Tfh/Tfc-like HIV-specific T cell populations, we identified CD8+ Tfc responses that were related to HIV plasma viral loads and associated with rate of antibody isotype class switching to IgG. This favorable balance towards IgG responses positively correlated with increased virus neutralization, higher avidity of neutralizing antibodies and more potent antibody-dependent cell cytotoxicity (ADCC) in PBMCs from HIV controllers compared to non-controllers. Our results identified the CD8+ Tfc-like T-cell response as a component of effective virus control which could possibly be exploited therapeutically.
Keywords: HIV; T-follicular cytotoxic (Tfc) cells; human immunodeficiency virus (HIV) control; humoral immune response; viral control.
Copyright © 2022 Romero-Martín, Tarrés-Freixas, Pedreño-López, de la Concepción, Cunyat, Hartigan-O'Connor, Carrillo, Mothe, Blanco, Ruiz-Riol, Brander and Olvera.
Conflict of interest statement
Author CB was employed by company AELIX Therapeutics. MR-R and CB are co-inventors of the “Boosted flow” technology, which is protected under patent application US9709577B2. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
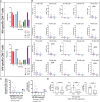
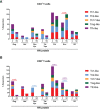
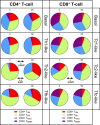



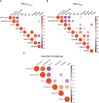

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

