Retrospective Comparison of Efficacy and Safety of Rabbit Anti-Thymocyte Globulin and Porcine Anti-Lymphocyte Globulin in Patients With Acquired Aplastic Anemia Undergoing Hematopoietic Stem Cell Transplantation From Matched Sibling Donors
- PMID: 35784311
- PMCID: PMC9241985
- DOI: 10.3389/fimmu.2022.889784
Retrospective Comparison of Efficacy and Safety of Rabbit Anti-Thymocyte Globulin and Porcine Anti-Lymphocyte Globulin in Patients With Acquired Aplastic Anemia Undergoing Hematopoietic Stem Cell Transplantation From Matched Sibling Donors
Abstract
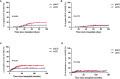
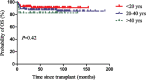
We compared the efficacy and safety of porcine anti-lymphocyte globulin (pALG) (n=140) and rabbit anti-thymocyte globulin (rATG) (n=86) in patients with acquired aplastic anemia (AA) receiving hematopoietic stem cell transplantation (HSCT) from matched sibling donors (MSD) in two transplantation centers in China ranging from 2005 to 2020. The groups had similar baseline characteristics except for a higher number of infused mononuclear cells (P<0.001) and a higher proportion of peripheral blood stem cells as graft sources (P=0.003) in the pALG group. The rates of neutrophil engraftment at day 28 (P=1), platelet engraftment at day 28 (P=0.228), bloodstream infection before engraftment (P=0.867), invasive fungal diseases (P=0.362), cytomegalovirus viremia (P=0.667), and graft rejection (P=0.147) were similar in the two groups. A higher cumulative incidence of grades II-IV acute graft versus host disease (aGvHD) at 100 days occurred in the pALG group (19% vs. 8%, P=0.035) while no significant differences in grades III-IV aGvHD (P=0.572), mild to severe chronic GvHD (cGvHD) (P=0.181), and moderate to severe cGvHD (P=0.586) were observed. The actuarial 5-year overall survival (OS), failure-free survival (FFS), and GvHD-free, FFS rates of the pALG group were 87% (95% confidence interval [CI], 82-93), 85% (95% CI, 80-92), and 78% (95% CI, 72-92) versus 91% (95% CI, 86-99) (P=0.33), 88% (95% CI, 82-97) (P=0.428), and 79% (95% CI, 72-90) (P=0.824) in the rATG group, respectively. A busulfan-containing conditioning regimen was the only adverse risk factor for OS and FFS in multivariate analysis. In conclusion, pALG is an alternative to rATG in patients with severe AA receiving MSD-HSCT. A prospective, large-sample study is needed to explore this therapy further.
Keywords: aplastic anemia; matched sibling donors; porcine; rabbit; stem cell transplantation.
Copyright © 2022 Zhang, Chen, Li, Li, Lin, Cao, Wang, Yang, Pang, Zhang, Ma, Zhai, He, Wei, Jiang, Han, Zhang and Feng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Cyclophosphamide and horse anti-thymocyte globulin versus fludarabine, reduced cyclophosphamide and rabbit anti-thymocyte globulin conditioning regimen for allogeneic hematopoietic stem cell transplantation from matched sibling donors in patients with acquired aplastic anemia.Expert Rev Hematol. 2024 Aug;17(8):527-538. doi: 10.1080/17474086.2024.2381572. Epub 2024 Jul 23. Expert Rev Hematol. 2024. PMID: 39011776
-
Comparison of Hematopoietic Stem Cell Transplantation Outcomes Using Matched Sibling Donors, Haploidentical Donors, and Immunosuppressive Therapy for Patients With Acquired Aplastic Anemia.Front Immunol. 2022 Feb 1;13:837335. doi: 10.3389/fimmu.2022.837335. eCollection 2022. Front Immunol. 2022. PMID: 35178053 Free PMC article.
-
[Clinical efficacy and safety of porcine antihuman lymphocyte immunoglobulin in alternative donor allogeneic hematopoietic cell transplantation for severe aplastic anemia].Zhonghua Xue Ye Xue Za Zhi. 2018 Jan 14;39(1):22-27. doi: 10.3760/cma.j.issn.0253-2727.2018.01.005. Zhonghua Xue Ye Xue Za Zhi. 2018. PMID: 29551028 Free PMC article. Chinese.
-
Porcine antilymphocyte globulin versus rabbit antithymocyte globulin for intensive immunosuppressive therapy of acquired aplastic anemia: A meta-analysis and systematic review.Int J Clin Pharmacol Ther. 2023 Dec;61(12):551-560. doi: 10.5414/CP204379. Int J Clin Pharmacol Ther. 2023. PMID: 37877292
-
Haploidentical hematopoietic stem cell transplantation in aplastic anemia: a systematic review and meta-analysis of clinical outcome on behalf of the severe aplastic anemia working party of the European group for blood and marrow transplantation (SAAWP of EBMT).Bone Marrow Transplant. 2020 Oct;55(10):1906-1917. doi: 10.1038/s41409-020-0897-2. Epub 2020 Apr 28. Bone Marrow Transplant. 2020. PMID: 32346079
Cited by
-
Early withdrawal immunosuppression improved mixed chimerism in stem cell transplantation for pediatric aplastic anemia.Int J Hematol. 2025 Aug;122(2):267-276. doi: 10.1007/s12185-025-03971-9. Epub 2025 May 23. Int J Hematol. 2025. PMID: 40408010
-
Porcine anti-human lymphocyte immunoglobulin depletes the lymphocyte population to promote successful kidney transplantation.Front Immunol. 2023 Mar 9;14:1124790. doi: 10.3389/fimmu.2023.1124790. eCollection 2023. Front Immunol. 2023. PMID: 36969156 Free PMC article.
References
-
- Bacigalupo A, Brand R, Oneto R, Bruno B, Socié G, Passweg J, et al. . Treatment of Acquired Severe Aplastic Anemia: Bone Marrow Transplantation Compared With Immunosuppressive Therapy–The European Group for Blood and Marrow Transplantation Experience. Semin Hematol (2000) 37(1):69–80. doi: 10.1016/s0037-1963(00)90031-3 - DOI - PubMed
-
- Locasciulli A, Oneto R, Bacigalupo A, Socié G, Korthof E, Bekassy A, et al. . Outcome of Patients With Acquired Aplastic Anemia Given First Line Bone Marrow Transplantation or Immunosuppressive Treatment in the Last Decade: A Report From the European Group for Blood and Marrow Transplantation (EBMT). Haematologica (2007) 92(1):11–8. doi: 10.3324/haematol.10075 - DOI - PubMed
-
- Yoshida N, Kobayashi R, Yabe H, Kosaka Y, Yagasaki H, Watanabe K, et al. . First-Line Treatment for Severe Aplastic Anemia in Children: Bone Marrow Transplantation From a Matched Family Donor Versus Immunosuppressive Therapy. Haematologica (2014) 99(12):1784–91. doi: 10.3324/haematol.2014.109355 - DOI - PMC - PubMed
-
- Dufour C, Pillon M, Sociè G, Rovò A, Carraro E, Bacigalupo A, et al. . Outcome of Aplastic Anaemia in Children. A Study by the Severe Aplastic Anaemia and Paediatric Disease Working Parties of the European Group Blood and Bone Marrow Transplant. Br J Haematol (2015) 169(4):565–73. doi: 10.1111/bjh.13297 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

