Mechanisms for Host Immune Evasion Mediated by Plasmodium falciparum-Infected Erythrocyte Surface Antigens
- PMID: 35784341
- PMCID: PMC9240312
- DOI: 10.3389/fimmu.2022.901864
Mechanisms for Host Immune Evasion Mediated by Plasmodium falciparum-Infected Erythrocyte Surface Antigens
Abstract
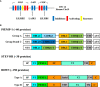
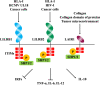
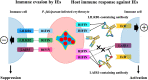
Plasmodium falciparum infection causes the most severe form of malaria. It has been hypothesized that P. falciparum directly suppresses host immune responses because sufficient acquired immunity is often not induced even by repeated P. falciparum infections in malaria-endemic areas. It is known that many kinds of P. falciparum-derived proteins are expressed on the surface of P. falciparum-infected erythrocytes (IEs), and these proteins have long been thought to be a key to the elucidation of the host immune evasion mechanisms. Our recent studies have revealed that the P. falciparum-derived erythrocyte surface antigen, RIFIN, the largest multiple gene family protein in the P. falciparum genome, suppresses host immune cell activation through direct interaction with human inhibitory immune receptors. In this review, we will discuss the molecular mechanisms for host immune evasion by P. falciparum-infected erythrocyte surface antigens. In addition, we will discuss the recently identified host immune response to P. falciparum using specialized antibodies that target host-P. falciparum-derived molecule interactions.
Keywords: Plasmodium falciparum; RIFIN; immune evasion; inhibitory immune receptors; receptor-containing antibodies; variant surface antigens.
Copyright © 2022 Sakoguchi and Arase.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- WHO. World Malaria Report 2021 . Geneva: World Health Organization; (2021). Available at: https://apps.who.int/iris/handle/10665/350147.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

