Dietary Mannan Oligosaccharides Enhance the Non-Specific Immunity, Intestinal Health, and Resistance Capacity of Juvenile Blunt Snout Bream (Megalobrama amblycephala) Against Aeromonas hydrophila
- PMID: 35784342
- PMCID: PMC9240629
- DOI: 10.3389/fimmu.2022.863657
Dietary Mannan Oligosaccharides Enhance the Non-Specific Immunity, Intestinal Health, and Resistance Capacity of Juvenile Blunt Snout Bream (Megalobrama amblycephala) Against Aeromonas hydrophila
Abstract
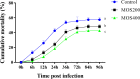
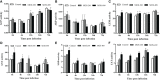
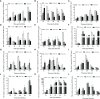
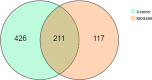
Mannan oligosaccharides (MOS) have been studied and applied as a feed additive, whereas their regulation on the growth performance and immunity of aquatic animals lacks consensus. Furthermore, their immunoprotective effects on the freshwater fish Megalobrama amblycephala have not been sufficiently studied. Thus, we investigated the effects of dietary MOS of 0, 200, and 400 mg/kg on the growth performance, non-specific immunity, intestinal health, and resistance to Aeromonas hydrophila infection in juvenile M. amblycephala. The results showed that the weight gain rate of juvenile M. amblycephala was not significantly different after 8 weeks of feeding, whereas the feed conversion ratio decreased in the MOS group of 400 mg/kg. Moreover, dietary MOS increased the survival rate of juvenile M. amblycephala upon infection, which may be attributed to enhanced host immunity. For instance, dietary MOS increase host bactericidal and antioxidative abilities by regulating the activities of hepatic antimicrobial and antioxidant enzymes. In addition, MOS supplementation increased the number of intestinal goblet cells, and the intestine was protected from necrosis of the intestinal folds and disruption of the microvilli and junctional complexes, thus maintaining the stability of the intestinal epithelial barrier. The expression levels of M. amblycephala immune and tight junction-related genes increased after feeding dietary MOS for 8 weeks. However, the upregulated expression of immune and tight junction-related genes in the MOS supplemental groups was not as notable as that in the control group postinfection. Therefore, MOS supplementation might suppress the damage caused by excessive intestinal inflammation. Furthermore, dietary MOS affected the richness and composition of the gut microbiota, which improved the gut health of juvenile M. amblycephala by increasing the relative abundance of beneficial gut microbiota. Briefly, dietary MOS exhibited significant immune protective effects to juvenile M. amblycephala, which is a functional feed additive and immunostimulant.
Keywords: Megalobrama amblycephala; immunoprotective effects; intestinal health; mannan oligosaccharides; non-specific immunity.
Copyright © 2022 Ding, Wang, Liu, Zheng, Li, Zhang, He, Cheng, Xu, Chen and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Li YS, San Andres JV, Trenhaile-Grannemann MD, van Sambeek DM, Moore KC, Winkel SM, et al. Effects of Mannan Oligosaccharides and Lactobacillus Mucosae on Growth Performance, Immune Response, and Gut Health of Weanling Pigs Challenged With Escherichia Coli Lipopolysaccharides. J Anim Sci (2021) 99(12):skab286. doi: 10.1093/jas/skab286 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

