As in Real Estate, Location Matters: Cellular Expression of Complement Varies Between Macular and Peripheral Regions of the Retina and Supporting Tissues
- PMID: 35784369
- PMCID: PMC9240314
- DOI: 10.3389/fimmu.2022.895519
As in Real Estate, Location Matters: Cellular Expression of Complement Varies Between Macular and Peripheral Regions of the Retina and Supporting Tissues
Abstract
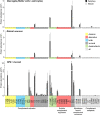
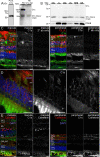
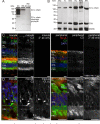
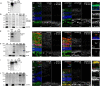
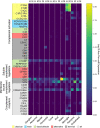
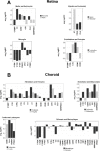
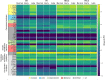
The cellular events that dictate the initiation of the complement pathway in ocular degeneration, such as age-related macular degeneration (AMD), is poorly understood. Using gene expression analysis (single cell and bulk), mass spectrometry, and immunohistochemistry, we dissected the role of multiple retinal and choroidal cell types in determining the complement homeostasis. Our scRNA-seq data show that the cellular response to early AMD is more robust in the choroid, particularly in fibroblasts, pericytes and endothelial cells. In late AMD, complement changes were more prominent in the retina especially with the expression of the classical pathway initiators. Notably, we found a spatial preference for these differences. Overall, this study provides insights into the heterogeneity of cellular responses for complement expression and the cooperation of neighboring cells to complete the pathway in healthy and AMD eyes. Further, our findings provide new cellular targets for therapies directed at complement.
Keywords: RPE/choroid; age-related macular degeneration; complement; retina; single cell.
Copyright © 2022 Zauhar, Biber, Jabri, Kim, Hu, Kaplan, Pfaller, Schäfer, Enzmann, Schlötzer-Schrehardt, Straub, Hauck, Gamlin, McFerrin, Messinger, Strang, Curcio, Dana, Pauly, Grosche, Li and Stambolian.
Conflict of interest statement
CC is a consultant for Apellis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

