The Role of Lamins in the Nucleoplasmic Reticulum, a Pleiomorphic Organelle That Enhances Nucleo-Cytoplasmic Interplay
- PMID: 35784476
- PMCID: PMC9243388
- DOI: 10.3389/fcell.2022.914286
The Role of Lamins in the Nucleoplasmic Reticulum, a Pleiomorphic Organelle That Enhances Nucleo-Cytoplasmic Interplay
Abstract
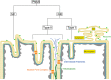
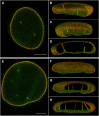
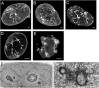
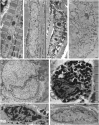
Invaginations of the nuclear membrane occur in different shapes, sizes, and compositions. Part of these pleiomorphic invaginations make up the nucleoplasmic reticulum (NR), while others are merely nuclear folds. We define the NR as tubular invaginations consisting of either both the inner and outer nuclear membrane, or only the inner nuclear membrane. Specifically, invaginations of both the inner and outer nuclear membrane are also called type II NR, while those of only the inner nuclear membrane are defined as type I NR. The formation and structure of the NR is determined by proteins associated to the nuclear membrane, which induce a high membrane curvature leading to tubular invaginations. Here we review and discuss the current knowledge of nuclear invaginations and the NR in particular. An increase in tubular invaginations of the nuclear envelope is associated with several pathologies, such as laminopathies, cancer, (reversible) heart failure, and Alzheimer's disease. Furthermore, viruses can induce both type I and II NR. In laminopathies, the amount of A-type lamins throughout the nucleus is generally decreased or the organization of lamins or lamin-associated proteins is disturbed. Also, lamin overexpression or modulation of lamin farnesylation status impacts NR formation, confirming the importance of lamin processing in NR formation. Virus infections reorganize the nuclear lamina via (de)phosphorylation of lamins, leading to an uneven thickness of the nuclear lamina and in turn lobulation of the nuclear membrane and the formation of invaginations of the inner nuclear membrane. Since most studies on the NR have been performed with cell cultures, we present additional proof for the existence of these structures in vivo, focusing on a variety of differentiated cardiovascular and hematopoietic cells. Furthermore, we substantiate the knowledge of the lamin composition of the NR by super-resolution images of the lamin A/C and B1 organization. Finally, we further highlight the essential role of lamins in NR formation by demonstrating that (over)expression of lamins can induce aberrant NR structures.
Keywords: STED microscopy; calcium regulation; electron microscopy; lamins; nuclear invaginations; nucleoplasmic reticulum.
Copyright © 2022 Stiekema, Houben, Verheyen, Borgers, Menzel, Meschkat, van Zandvoort, Ramaekers and Broers.
Conflict of interest statement
JM and MM were employed by Abberior Instruments GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Mechanisms for assembly of the nucleoplasmic reticulum.Cell Mol Life Sci. 2024 Oct 5;81(1):415. doi: 10.1007/s00018-024-05437-3. Cell Mol Life Sci. 2024. PMID: 39367888 Free PMC article. Review.
-
CTP:phosphocholine cytidylyltransferase α (CCTα) and lamins alter nuclear membrane structure without affecting phosphatidylcholine synthesis.Biochim Biophys Acta. 2011 Jun;1811(6):377-85. doi: 10.1016/j.bbalip.2011.04.001. Epub 2011 Apr 9. Biochim Biophys Acta. 2011. PMID: 21504799
-
Formation of a nucleoplasmic reticulum requires de novo assembly of nascent phospholipids and shows preferential incorporation of nascent lamins.Sci Rep. 2017 Aug 7;7(1):7454. doi: 10.1038/s41598-017-07614-w. Sci Rep. 2017. PMID: 28785031 Free PMC article.
-
Organization and modulation of nuclear lamina structure.J Cell Sci Suppl. 1984;1:137-60. doi: 10.1242/jcs.1984.supplement_1.10. J Cell Sci Suppl. 1984. PMID: 6597817
-
Nuclear lamin phosphorylation: an emerging role in gene regulation and pathogenesis of laminopathies.Nucleus. 2020 Dec;11(1):299-314. doi: 10.1080/19491034.2020.1832734. Nucleus. 2020. PMID: 33030403 Free PMC article. Review.
Cited by
-
Mechanisms for assembly of the nucleoplasmic reticulum.Cell Mol Life Sci. 2024 Oct 5;81(1):415. doi: 10.1007/s00018-024-05437-3. Cell Mol Life Sci. 2024. PMID: 39367888 Free PMC article. Review.
-
Grease in the Nucleus: Insights into the Dynamic Life of Nuclear Membranes.J Membr Biol. 2023 Apr;256(2):137-145. doi: 10.1007/s00232-022-00272-8. Epub 2022 Nov 4. J Membr Biol. 2023. PMID: 36331589 Free PMC article. Review.
-
Nucleus Mechanosensing in Cardiomyocytes.Int J Mol Sci. 2023 Aug 28;24(17):13341. doi: 10.3390/ijms241713341. Int J Mol Sci. 2023. PMID: 37686151 Free PMC article. Review.
-
The destruction of cytoplasmic skeleton leads to the change of nuclear structure and the looseness of lamin A submicroscopic network.Heliyon. 2024 Sep 7;10(18):e36583. doi: 10.1016/j.heliyon.2024.e36583. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309767 Free PMC article.
-
Observing bioorthogonal macrocyclizations in the nuclear envelope of live cells using on/on fluorescence lifetime microscopy.Chem Sci. 2024 Aug 20;15(36):14913-23. doi: 10.1039/d4sc03489a. Online ahead of print. Chem Sci. 2024. PMID: 39184298 Free PMC article.
References
-
- Ancla M., de Brux J., Bret J. (1965). Action of Clomiphene on the Endometrium. Study by Optic and Electron Microscopes. Gynecol. Obstet. Paris. 64, 611–620. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

