Nonclinical Bench Performance Testing of a Very Low-Cost Nonelectric Bubble Continuous Positive Airway Pressure (bCPAP) and Blenders Device Designed for Newborn Respiratory Support
- PMID: 35784612
- PMCID: PMC9249094
- DOI: 10.2147/MDER.S318218
Nonclinical Bench Performance Testing of a Very Low-Cost Nonelectric Bubble Continuous Positive Airway Pressure (bCPAP) and Blenders Device Designed for Newborn Respiratory Support
Abstract
Purpose: Bubble continuous positive airway pressure (bCPAP) is often used to treat respiratory distress experienced by some 15 million preterm infants born globally every year. In low- and middle-income countries, improvised bCPAP devices are used, often without a blender that protects the infant from the sequelae of excessive oxygen exposure.
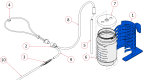
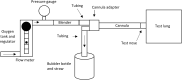
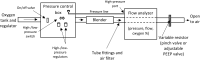
Materials and methods: The aim of this bench testing was to assess the mechanical safety and performance of the PATH bCPAP and blenders device, which provides a stable and reliable source of pressurized blended gas without the requirement for a source of compressed medical air or electricity. The device includes two fixed ratio blenders: a "low" blend that provides 37% oxygen and a "high" blend that provides 60% oxygen. We performed bench testing to characterize the performance of the bCPAP and blenders, including respiratory circuit verification, blender verification, conditioned humidity testing, and sound measurement.
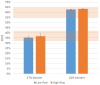
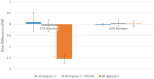
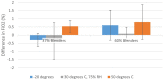
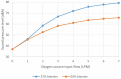
Results: Test results for all performance variables met the acceptance criteria of our product requirement specification. The device provides a fixed ratio of air and oxygen that is consistent over the entire range of clinically relevant pressures (4 to 8 cmH2O) and remains consistent despite changes in flow (2 to 7 liters per minute). The blend is stable within ± 5% of the blenders' nominal blend ratio when used with a 100% oxygen source, irrespective of the flow and pressure from the oxygen source or the flow and pressure of the blended gas delivered to the neonate. Sound and humidity test results were within specifications.
Conclusion: This very low-cost nonelectric bCPAP and blenders device is optimally designed to deliver a stable and reliable source of pressurized blended gas.
Keywords: affordability; biomedical engineering; global health; hyperoxia; medical device design; noninvasive ventilation; preterm infants; respiratory distress syndrome.
© 2022 Coffey and Wollen.
Conflict of interest statement
Both authors have been active in developing the bCPAP and blenders technology while employed at PATH. PATH and/or the authors do not hold any relevant financial or nonfinancial relationships related to this product. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
Feasibility and usability of a very low-cost bubble continuous positive airway pressure device including oxygen blenders in a Ugandan level two newborn unit.PLOS Glob Public Health. 2023 Mar 8;3(3):e0001354. doi: 10.1371/journal.pgph.0001354. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 36963078 Free PMC article.
-
A new low-cost commercial bubble CPAP (bCPAP) machine compared with a traditional bCPAP device in Nigeria.Paediatr Int Child Health. 2019 Aug;39(3):184-192. doi: 10.1080/20469047.2019.1598125. Epub 2019 Apr 8. Paediatr Int Child Health. 2019. PMID: 30957682
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
To Bubble or Not? A Systematic Review of Bubble Continuous Positive Airway Pressure in Children in Low- and Middle-Income Countries.J Trop Pediatr. 2020 Jun 1;66(3):339-353. doi: 10.1093/tropej/fmz069. J Trop Pediatr. 2020. PMID: 31599959
-
Bubble CPAP devices for infants and children in resource-limited settings: review of the literature.Paediatr Int Child Health. 2019 Aug;39(3):168-176. doi: 10.1080/20469047.2018.1534389. Epub 2018 Oct 30. Paediatr Int Child Health. 2019. PMID: 30375281
Cited by
-
Evaluation of an innovative low flow oxygen blender system for global access.Front Pediatr. 2022 Sep 12;10:981821. doi: 10.3389/fped.2022.981821. eCollection 2022. Front Pediatr. 2022. PMID: 36186642 Free PMC article.
-
Respiratory distress syndrome management in resource limited settings-Current evidence and opportunities in 2022.Front Pediatr. 2022 Jul 29;10:961509. doi: 10.3389/fped.2022.961509. eCollection 2022. Front Pediatr. 2022. PMID: 35967574 Free PMC article. Review.
-
Feasibility and usability of a very low-cost bubble continuous positive airway pressure device including oxygen blenders in a Ugandan level two newborn unit.PLOS Glob Public Health. 2023 Mar 8;3(3):e0001354. doi: 10.1371/journal.pgph.0001354. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 36963078 Free PMC article.
References
-
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. doi:10.1016/S0140-6736(12)60820-4 - DOI - PubMed
-
- World Health Organization. Preterm birth fact sheet. Available from: http://www.who.int/mediacentre/factsheets/fs363/en/. Accessed January 24, 2022.
LinkOut - more resources
Full Text Sources
Miscellaneous

