Identification of the stress granule transcriptome via RNA-editing in single cells and in vivo
- PMID: 35784648
- PMCID: PMC9243631
- DOI: 10.1016/j.crmeth.2022.100235
Identification of the stress granule transcriptome via RNA-editing in single cells and in vivo
Abstract
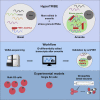
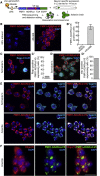
Stress granules are phase-separated assemblies formed around RNAs. So far, the techniques available to identify these RNAs are not suitable for single cells and small tissues displaying cell heterogeneity. Here, we used TRIBE (target of RNA-binding proteins identified by editing) to profile stress granule RNAs. We used an RNA-binding protein (FMR1) fused to the catalytic domain of an RNA-editing enzyme (ADAR), which coalesces into stress granules upon oxidative stress. RNAs colocalized with this fusion are edited, producing mutations that are detectable by VASA sequencing. Using single-molecule FISH, we validated that this purification-free method can reliably identify stress granule RNAs in bulk and single S2 cells and in Drosophila neurons. Similar to mammalian cells, we find that stress granule mRNAs encode ATP binding, cell cycle, and transcription factors. This method opens the possibility to identify stress granule RNAs and other RNA-based assemblies in other single cells and tissues.
Keywords: Drosophila; RNA; RNA-editing; S2 cells; VASA-seq; hyperTRIBE; neurons; single cell; stress granules.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Aguilera-Gomez A., Zacharogianni M., Van Oorschot M.M., Genau H., Grond R., Veenendaal T., Sinsimer K.S., Gavis E.R., Behrends C., Rabouille C. Phospho-rasputin stabilization by Sec16 is required for stress granule formation upon amino acid starvation. Cell Rep. 2017;20:935–948. doi: 10.1016/j.celrep.2017.06.042. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

