Development of an HIV reporter virus that identifies latently infected CD4+ T cells
- PMID: 35784650
- PMCID: PMC9243624
- DOI: 10.1016/j.crmeth.2022.100238
Development of an HIV reporter virus that identifies latently infected CD4+ T cells
Abstract
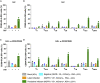
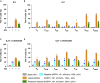
There is no cure for HIV infection, as the virus establishes a latent reservoir, which escapes highly active antiretroviral treatments. One major obstacle is the difficulty identifying cells that harbor latent proviruses. We devised a single-round viral vector that carries a series of versatile reporter molecules that are expressed in an LTR-dependent or LTR-independent manner and make it possible to accurately distinguish productive from latent infection. Using primary human CD4+ T cells, we show that transcriptionally silent proviruses are found in more than 50% of infected cells. The latently infected cells harbor proviruses but lack evidence for multiple spliced transcripts. LTR-silent integrations occurred to variable degrees in all CD4+ T subsets examined, with CD4+ TEM and CD4+ TREG displaying the highest frequency of latent infections. This viral vector permits the interrogation of HIV latency at single-cell resolution, revealing mechanisms of latency establishment and allowing the characterization of effective latency-reversing agents.
Keywords: CD4+ T memory cells; CD4+ T stem cells; CD4+ T cell subsets; HIV integration; HIV persistence; HIV reservoir; HIV-1; latency establishment; mass cytometry; reporter virus.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Amir E.a.D., Davis K.L., Tadmor M.D., Simonds E.F., Levine J.H., Bendall S.C., Shenfeld D.K., Krishnaswamy S., Nolan G.P., Pe'er D. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol. 2013;31:545–552. doi: 10.1038/nbt.2594. - DOI - PMC - PubMed
-
- Bacchus C., Cheret A., Avettand-Fenoel V., Nembot G., Melard A., Blanc C., Lascoux-Combe C., Slama L., Allegre T., Allavena C., et al. A single HIV-1 cluster and a skewed immune homeostasis drive the early spread of HIV among resting CD4+ cell subsets within one month post-infection. PLoS One. 2013;8:e64219. doi: 10.1371/journal.pone.0064219. - DOI - PMC - PubMed
-
- Battivelli E., Dahabieh M.S., Abdel-Mohsen M., Svensson J.P., Tojal Da Silva I., Cohn L.B., Gramatica A., Deeks S., Greene W.C., Pillai S.K., Verdin E. Distinct chromatin functional states correlate with HIV latency reactivation in infected primary CD4(+) T cells. Elife. 2018;7 doi: 10.7554/elife.34655. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

