The Effects of a Transgelin-2 Agonist Administered at Different Times in a Mouse Model of Airway Hyperresponsiveness
- PMID: 35784706
- PMCID: PMC9243334
- DOI: 10.3389/fphar.2022.873612
The Effects of a Transgelin-2 Agonist Administered at Different Times in a Mouse Model of Airway Hyperresponsiveness
Abstract
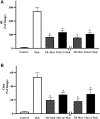
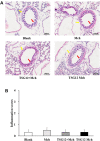
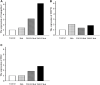
Airway hyperresponsiveness (AHR) is one of the most important features of asthma. Our previous study showed that inhaled transgelin-2 agonist, TSG12, effectively reduced pulmonary resistance in a mouse model of asthma in a dose-dependent manner. However, the optimal administration time of TSG12 to reduce AHR and the pharmacological effects are still unclear. In this study, the effects of TSG12 inhalation before and during AHR occurrence were examined. The results showed that the pulmonary resistance was reduced by 57% and the dynamic compliance was increased by 46% in the TSG12 Mch group (atomize TSG12 10 min before methacholine, p < 0.05 vs. model). The pulmonary resistance was reduced by 61% and the dynamic compliance was increased by 47% in the TSG12 + Mch group (atomize TSG12 and methacholine together, p < 0.05 vs. model). Quantitative real-time PCR showed that the gene expression levels of transgelin-2, myosin phosphatase target subunit-1, and myosin light chain were up-regulated by 6.4-, 1.9-, and 2.8-fold, respectively, in the TSG12 Mch group. The gene expression levels of transgelin-2, myosin phosphatase target subunit-1, and myosin light chain were up-regulated by 3.2-, 1.4-, and 1.9-fold, respectively, in the TSG12 + Mch group. The results suggested that TSG12 effectively reduces pulmonary resistance when TSG12 inhalation occurred both before and during AHR occurrence. Gene expression levels of transgelin-2 and myosin light chain were significantly up-regulated when TSG12 inhalation occurred before AHR occurrence. This study may provide a basis for the administration time of TSG12 for asthma treatment in the future.
Keywords: TSG12; administration time; agonist; airway hyperresponsiveness; asthma; transgelin-2.
Copyright © 2022 Yuan, Lu, Wang, Lv, Li, Zhu, Yang and Yin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Molecular dynamics study on the behavior and binding mechanism of target protein Transgelin-2 with its agonist TSG12 for anti-asthma drug discovery.Comput Biol Med. 2023 Feb;153:106515. doi: 10.1016/j.compbiomed.2022.106515. Epub 2022 Dec 31. Comput Biol Med. 2023. PMID: 36610217
-
Transgelin-2 as a therapeutic target for asthmatic pulmonary resistance.Sci Transl Med. 2018 Feb 7;10(427):eaam8604. doi: 10.1126/scitranslmed.aam8604. Sci Transl Med. 2018. PMID: 29437149 Free PMC article.
-
Discovery of zolinium TSG1180 as a novel agonist of transgelin-2 for treating asthma.Biomed Pharmacother. 2023 Nov;167:115556. doi: 10.1016/j.biopha.2023.115556. Epub 2023 Sep 29. Biomed Pharmacother. 2023. PMID: 37778269
-
Transgelin-2: Biochemical and Clinical Implications in Cancer and Asthma.Trends Biochem Sci. 2019 Oct;44(10):885-896. doi: 10.1016/j.tibs.2019.05.004. Epub 2019 Jun 27. Trends Biochem Sci. 2019. PMID: 31256982 Free PMC article. Review.
-
Mechanisms of airway hyperresponsiveness.J Allergy Clin Immunol. 2006 Sep;118(3):551-9; quiz 560-1. doi: 10.1016/j.jaci.2006.07.012. J Allergy Clin Immunol. 2006. PMID: 16950269 Review.
Cited by
-
Micro-simulation insights into the functional and mechanistic understanding of glycyrrhizin against asthma.Front Pharmacol. 2023 Aug 28;14:1220368. doi: 10.3389/fphar.2023.1220368. eCollection 2023. Front Pharmacol. 2023. PMID: 37711178 Free PMC article.
-
RhoA/ROCK/GSK3β Signaling: A Keystone in Understanding Alzheimer's Disease.Curr Issues Mol Biol. 2025 Feb 14;47(2):124. doi: 10.3390/cimb47020124. Curr Issues Mol Biol. 2025. PMID: 39996845 Free PMC article. Review.
-
Trends of therapy in the treatment of asthma.Ther Adv Respir Dis. 2023 Jan-Dec;17:17534666231155748. doi: 10.1177/17534666231155748. Ther Adv Respir Dis. 2023. PMID: 36942731 Free PMC article. Review.
References
-
- Asthma and Allergy Foundation of America (2021). 2021 Asthma Capitals. Availableat: https://www.aafa.org/asthma-capitals (Accessed June 26, 2021).
LinkOut - more resources
Full Text Sources

