Reimbursed Medication Adherence Enhancing Interventions in European Countries: Results of the EUREcA Study
- PMID: 35784711
- PMCID: PMC9247400
- DOI: 10.3389/fphar.2022.892240
Reimbursed Medication Adherence Enhancing Interventions in European Countries: Results of the EUREcA Study
Abstract
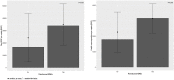
Introduction: Current literature lacks detailed understanding of the reimbursement framework of medication adherence enhancing interventions (MAEIs). As part of the ENABLE COST Action, the EUREcA ("EUropen REimbursement strategies for interventions targeting medication Adherence") study aimed to provide an in-depth overview of reimbursed MAEIs currently available in European countries at national and regional levels and to pave the way for further MAEIs to be implemented in the future. Methods: A web-based, cross-sectional survey was performed across 38 European countries and Israel. The survey questionnaire was developed as a result of an iterative process of discussion informed by a desk review. The survey was performed among invited ENABLE collaborators from June to July 2021. Besides descriptive analysis, association between country income and health care expenditure, and the availability of reimbursed MAEIs were also assessed. Results: The survey identified 13 reimbursed MAEIs in nine countries: multi-dose drug dispensing (n = 5), medication review (n = 4), smart device (n = 2), mobile application (n = 1), and patient education (n = 1). The median GDP per capita of countries having ≥1 reimbursed MAEI was significantly higher compared to countries having no reimbursed adherence intervention (33,888 EUR vs 16,620 EUR, respectively; p = 0.05). Conclusions: Our findings highlight that to date only a small number of MAEIs have been reimbursed in European countries. Comprehensive health technology assessment recommendations and multi-stakeholder collaboration could help removing barriers related to the implementation and reimbursement of MAEIs.
Keywords: health economics; health policy; intervention; medication adherence; persistence; reimbursement.
Copyright © 2022 Ágh, Hadžiabdić, Garuoliene, Granas, Aarnio, Menditto, Gregório, Barnestein-Fonseca, Mevsim and Kardas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ágh T., van Boven J. F., Wettermark B., Menditto E., Pinnock H., Tsiligianni I., et al. (2021). A Cross-Sectional Survey on Medication Management Practices for Noncommunicable Diseases in Europe during the Second Wave of the COVID-19 Pandemic. Front. Pharmacol. 12, 685696. 10.3389/fphar.2021.685696 - DOI - PMC - PubMed
-
- Breekveldt-Postma N. S., Penning-van Beest F. J., Siiskonen S. J., Falvey H., Vincze G., Klungel O. H., et al. (2008). The Effect of Discontinuation of Antihypertensives on the Risk of Acute Myocardial Infarction and Stroke. Curr. Med. Res. Opin. 24 (1), 121–127. 10.1185/030079908x253843 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

