SARS-CoV-2 accelerated clearance using a novel nitric oxide nasal spray (NONS) treatment: A randomized trial
- PMID: 35784831
- PMCID: PMC9239922
- DOI: 10.1016/j.lansea.2022.100036
SARS-CoV-2 accelerated clearance using a novel nitric oxide nasal spray (NONS) treatment: A randomized trial
Erratum in
-
Correction to "SARS-CoV-2 accelerated clearance using a novel nitric oxide nasal spray (NONS) treatment: A randomized trial".Lancet Reg Health Southeast Asia. 2022 Dec;7:100110. doi: 10.1016/j.lansea.2022.100110. Epub 2022 Nov 6. Lancet Reg Health Southeast Asia. 2022. PMID: 36373109 Free PMC article.
Abstract
Background: Additional outpatient therapies which are readily accessible will be essential to reduce COVID-19 illness progression in high risk individuals. Especially as the virus continues to mutate with greater transmissibility despite increased global vaccination.
Methods: A randomized, double-blind, multicentre, parallel group, placebo-controlled phase III clinical trial evaluated the ability of nitric oxide (NO) to rapidly eradicate nasal SARS-CoV-2 RNA. Adults (18-70 years) with mild symptomatic COVID-19 were randomized, confirmed by laboratory SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) nasal swab. Randomisation was 1:1, NONS (N = 153) vs placebo (N = 153). NO generated by a nasal spray (NONS) was self-administered six times daily as two sprays per nostril (0⋅45 mL of solution/dose) for seven days. Patients at high risk of illness progression, defined as unvaccinated, ≥ 45 years of age or having comorbidities, were the primary analysis population.
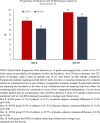
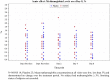
Findings: Overall, mean SARS-CoV-2 RNA concentrations (6·96 log10 copies/mL in the NONS group and 7·16 log10 copies/mL in the placebo group) were comparable at baseline. Primary endpoint mean treatment difference SARS-CoV-2 RNA change from baseline to the end of treatment (EOT) was -0·52 copies/mL (SE 0·202, 95% CI -0·92 to -0·12; p = 0·010) with NONS compared to placebo. Secondary endpoint assessments demonstrated a greater proportion of patients receiving NONS (82·8%) cleared SARS-CoV-2 (RT-PCR negative) by EOT compared to placebo (66·7%, p = 0·046), with no virus RNA detected a median of four days earlier compared to placebo (three vs seven days; p = 0·044).
Interpretation: Use of NONS in patients recently infected with SARS-CoV-2 accelerates nasal virus clearance.
Funding: Funding provided by Glenmark Pharmaceuticals Limited. Study medication provided by SaNOtize.
Keywords: Anti-viral treatment; COVID-19; Nasal Spray; SARS-CoV-2; Viral load.
© 2022 The Authors.
Conflict of interest statement
We declare no competing interests.
Figures



Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Clinical efficacy of nitric oxide nasal spray (NONS) for the treatment of mild COVID-19 infection.J Infect. 2021 Aug;83(2):237-279. doi: 10.1016/j.jinf.2021.05.009. Epub 2021 May 13. J Infect. 2021. PMID: 33992687 Free PMC article.
-
The SARS-CoV-2 Ivermectin Navarra-ISGlobal Trial (SAINT) to Evaluate the Potential of Ivermectin to Reduce COVID-19 Transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: A structured summary of a study protocol for a randomized control pilot trial.Trials. 2020 Jun 8;21(1):498. doi: 10.1186/s13063-020-04421-z. Trials. 2020. PMID: 32513289 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Randomized clinical trial to compare the efficacy of ivermectin versus placebo to negativize nasopharyngeal PCR in patients with early COVID-19 in Peru (SAINT-Peru): a structured summary of a study protocol for randomized controlled trial.Trials. 2021 Apr 9;22(1):262. doi: 10.1186/s13063-021-05236-2. Trials. 2021. PMID: 33836826 Free PMC article.
Cited by
-
Nitric Oxide Signaling and Sensing in Age-Related Diseases.Antioxidants (Basel). 2024 Oct 9;13(10):1213. doi: 10.3390/antiox13101213. Antioxidants (Basel). 2024. PMID: 39456466 Free PMC article. Review.
-
High-Dose Inhaled Nitric Oxide in Acute Hypoxemic Respiratory Failure Due to COVID-19: A Multicenter Phase II Trial.Am J Respir Crit Care Med. 2023 Dec 15;208(12):1293-1304. doi: 10.1164/rccm.202304-0637OC. Am J Respir Crit Care Med. 2023. PMID: 37774011 Free PMC article. Clinical Trial.
-
Nitric Oxide Therapeutics: New Hopes for More Effective Tuberculosis Treatment Combine with Targeted and Controlled Nanotechnology.Int J Nanomedicine. 2025 Jul 19;20:9195-9218. doi: 10.2147/IJN.S531255. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40703475 Free PMC article. Review.
-
Real-world effectiveness of an intranasal spray A8G6 antibody cocktail in the post-exposure prophylaxis of COVID-19.Signal Transduct Target Ther. 2023 Oct 23;8(1):403. doi: 10.1038/s41392-023-01656-5. Signal Transduct Target Ther. 2023. PMID: 37867182 Free PMC article. Clinical Trial.
-
Azelastine Nasal Spray in Non-Hospitalized Subjects with Mild COVID-19 Infection: A Randomized Placebo-Controlled, Parallel-Group, Multicentric, Phase II Clinical Trial.Viruses. 2024 Dec 13;16(12):1914. doi: 10.3390/v16121914. Viruses. 2024. PMID: 39772221 Free PMC article. Clinical Trial.
References
-
- World Health Organization. Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed 1 March 2022
-
- Moncada S, Higgs E. Endogenous nitric oxide: physiology, pathology, and clinical relevance. Eur J Clin Invest. 1991;21(4):361–374. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

