Potential for Ketotherapies as Amyloid-Regulating Treatment in Individuals at Risk for Alzheimer's Disease
- PMID: 35784855
- PMCID: PMC9243383
- DOI: 10.3389/fnins.2022.899612
Potential for Ketotherapies as Amyloid-Regulating Treatment in Individuals at Risk for Alzheimer's Disease
Abstract
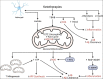
Alzheimer's disease (AD) is a progressive neurodegenerative condition characterized by clinical decline in memory and other cognitive functions. A classic AD neuropathological hallmark includes the accumulation of amyloid-β (Aβ) plaques, which may precede onset of clinical symptoms by over a decade. Efforts to prevent or treat AD frequently emphasize decreasing Aβ through various mechanisms, but such approaches have yet to establish compelling interventions. It is still not understood exactly why Aβ accumulates in AD, but it is hypothesized that Aβ and other downstream pathological events are a result of impaired bioenergetics, which can also manifest prior to cognitive decline. Evidence suggests that individuals with AD and at high risk for AD have functional brain ketone metabolism and ketotherapies (KTs), dietary approaches that produce ketone bodies for energy metabolism, may affect AD pathology by targeting impaired brain bioenergetics. Cognitively normal individuals with elevated brain Aβ, deemed "preclinical AD," and older adults with peripheral metabolic impairments are ideal candidates to test whether KTs modulate AD biology as they have impaired mitochondrial function, perturbed brain glucose metabolism, and elevated risk for rapid Aβ accumulation and symptomatic AD. Here, we discuss the link between brain bioenergetics and Aβ, as well as the potential for KTs to influence AD risk and progression.
Keywords: Alzheimer’s disease; amyloid; exogenous ketones; ketogenic diet; ketotherapy; medium chain triglyceride (MCT); mitochondria.
Copyright © 2022 Taylor, Sullivan, Keller, Burns and Swerdlow.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article.
-
Sensitivity of a Preclinical Alzheimer's Cognitive Composite (PACC) to amyloid β load in preclinical Alzheimer's disease.J Clin Exp Neuropsychol. 2019 Aug;41(6):591-600. doi: 10.1080/13803395.2019.1593949. Epub 2019 Mar 29. J Clin Exp Neuropsychol. 2019. PMID: 30924399
-
2-Deoxy-D-glucose treatment induces ketogenesis, sustains mitochondrial function, and reduces pathology in female mouse model of Alzheimer's disease.PLoS One. 2011;6(7):e21788. doi: 10.1371/journal.pone.0021788. Epub 2011 Jul 1. PLoS One. 2011. PMID: 21747957 Free PMC article.
-
Ketogenic Diet as a Promising Non-Drug Intervention for Alzheimer's Disease: Mechanisms and Clinical Implications.J Alzheimers Dis. 2023;92(4):1173-1198. doi: 10.3233/JAD-230002. J Alzheimers Dis. 2023. PMID: 37038820 Review.
Cited by
-
Creatine as a Therapeutic Target in Alzheimer's Disease.Curr Dev Nutr. 2023 Sep 29;7(11):102011. doi: 10.1016/j.cdnut.2023.102011. eCollection 2023 Nov. Curr Dev Nutr. 2023. PMID: 37881206 Free PMC article. Review.
-
Nifedipine Improves the Ketogenic Diet Effect on Insulin-Resistance-Induced Cognitive Dysfunction in Rats.Pharmaceuticals (Basel). 2024 Aug 10;17(8):1054. doi: 10.3390/ph17081054. Pharmaceuticals (Basel). 2024. PMID: 39204160 Free PMC article.
-
Protocol for a single-arm, pilot trial of creatine monohydrate supplementation in patients with Alzheimer's disease.Pilot Feasibility Stud. 2024 Feb 27;10(1):42. doi: 10.1186/s40814-024-01469-5. Pilot Feasibility Stud. 2024. PMID: 38414003 Free PMC article.
-
The ketone body β-hydroxybutyrate shifts microglial metabolism and suppresses amyloid-β oligomer-induced inflammation in human microglia.FASEB J. 2023 Nov;37(11):e23261. doi: 10.1096/fj.202301254R. FASEB J. 2023. PMID: 37878335 Free PMC article.
-
The impact of mild episodic ketosis on microglia and hippocampal long-term depression in 5xFAD mice.FASEB Bioadv. 2024 Oct 23;6(12):581-596. doi: 10.1096/fba.2024-00123. eCollection 2024 Dec. FASEB Bioadv. 2024. PMID: 39650227 Free PMC article.
References
-
- Apfelbaum M., Lacatis D., Reinberg A., Assan R. (1972). Persisting circadian rhythm in insulin, glucagon, cortisol etc. of healthy young women during caloric restriction (protein diet). Rev. Med. Chir. Soc. Med. Nat. Iasi. 76 123–130. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

