Mechanosensitive Hydrolysis of ATP and ADP in Lamina Propria of the Murine Bladder by Membrane-Bound and Soluble Nucleotidases
- PMID: 35784885
- PMCID: PMC9246094
- DOI: 10.3389/fphys.2022.918100
Mechanosensitive Hydrolysis of ATP and ADP in Lamina Propria of the Murine Bladder by Membrane-Bound and Soluble Nucleotidases
Abstract
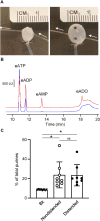
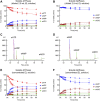
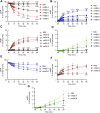
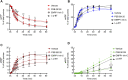
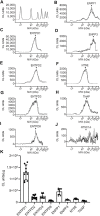
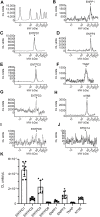
Prior studies suggest that urothelium-released adenosine 5'-triphosphate (ATP) has a prominent role in bladder mechanotransduction. Urothelial ATP regulates the micturition cycle through activation of purinergic receptors that are expressed in many cell types in the lamina propria (LP), including afferent neurons, and might also be important for direct mechanosensitive signaling between urothelium and detrusor. The excitatory action of ATP is terminated by enzymatic hydrolysis, which subsequently produces bioactive metabolites. We examined possible mechanosensitive mechanisms of ATP hydrolysis in the LP by determining the degradation of 1,N 6 -etheno-ATP (eATP) at the anti-luminal side of nondistended (empty) or distended (full) murine (C57BL/6J) detrusor-free bladder model, using HPLC. The hydrolysis of eATP and eADP was greater in contact with LP of distended than of nondistended bladders whereas the hydrolysis of eAMP remained unchanged during filling, suggesting that some steps of eATP hydrolysis in the LP are mechanosensitive. eATP and eADP were also catabolized in extraluminal solutions (ELS) that were in contact with the LP of detrusor-free bladders, but removed from the organ chambers prior to addition of substrate. The degradation of both purines was greater in ELS from distended than from nondistended preparations, suggesting the presence of mechanosensitive release of soluble nucleotidases in the LP. The released enzyme activities were affected differently by Ca2+ and Mg2+. The common nucleotidase inhibitors ARL67156, POM-1, PSB06126, and ENPP1 Inhibitor C, but not the alkaline phosphatase inhibitor (-)-p-bromotetramisole oxalate, inhibited the enzymes released during bladder distention. Membrane-bound nucleotidases were identified in tissue homogenates and in concentrated ELS from distended preparations by Wes immunodetection. The relative distribution of nucleotidases was ENTPD1 >> ENPP1 > ENTPD2 = ENTPD3 > ENPP3 = NT5E >> ENTPD8 = TNAP in urothelium and ENTPD1 >> ENTPD3 >> ENPP3 > ENPP1 = ENTPD2 = NT5E >> ENTPD8 = TNAP in concentrated ELS, suggesting that regulated ectodomain shedding of membrane-bound nucleotidases possibly occurs in the LP during bladder filling. Mechanosensitive degradation of ATP and ADP by membrane-bound and soluble nucleotidases in the LP diminishes the availability of excitatory purines in the LP at the end of bladder filling. This might be a safeguard mechanism to prevent over-excitability of the bladder. Proper proportions of excitatory and inhibitory purines in the bladder wall are determined by distention-associated purine release and purine metabolism.
Keywords: ADP; ATP hydrolysis; ATP-adenosine triphosphate; bladder; lamina propria (LP); nucleotidases; purine nucleotides; urothelium.
Copyright © 2022 Aresta Branco, Gutierrez Cruz, Dayton, Perrino and Mutafova-Yambolieva.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Abbracchio M. P., Burnstock G., Boeynaems J.-M., Barnard E. A., Boyer J. L., Kennedy C., et al. (2006). International Union of Pharmacology LVIII: Update on the P2Y G Protein-Coupled Nucleotide Receptors: from Molecular Mechanisms and Pathophysiology to Therapy. Pharmacol. Rev. 58 (3), 281–341. 10.1124/pr.58.3.3 - DOI - PMC - PubMed
-
- Baqi Y., Weyler S., Iqbal J., Zimmermann H., Müller C. E. (2009). Structure-activity Relationships of Anthraquinone Derivatives Derived from Bromaminic Acid as Inhibitors of Ectonucleoside Triphosphate Diphosphohydrolases (E-NTPDases). Purinergic Signal. 5 (1), 91–106. 10.1007/s11302-008-9103-5 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

