Physiological Responses of the Firefly Pyrocoelia analis (Coleoptera: Lampyridae) to an Environmental Residue From Chemical Pesticide Imidacloprid
- PMID: 35784886
- PMCID: PMC9240607
- DOI: 10.3389/fphys.2022.879216
Physiological Responses of the Firefly Pyrocoelia analis (Coleoptera: Lampyridae) to an Environmental Residue From Chemical Pesticide Imidacloprid
Abstract
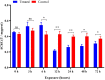
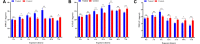
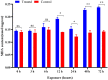
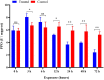
Imidacloprid, a neonicotinoid insecticide, is widely applied to control insect pests across a broad spectrum. Though the impact of residues from this chemical pesticide on non-target organisms in the field has been reported, it was not well characterized across a wide range of ecosystems, especially for some species considered as environmental indicators that live in forests. The effects of sublethal dose of imidacloprid on firefly, Pyrocoelia analis, were analyzed physiologically and biochemically in this study to better understand the impact of chemical pesticide application on environmental indicators such as fireflies. After imidacloprid treatment, the midgut tissues of the larva presented an abnormal morphology featured as atrophy of fat body cells, shrinking cells, and the destruction of a midgut structure. The activities of antioxidant enzymes, superoxide dismutase, catalase, and peroxidase were noticeably increased during early exposure to sublethal imidacloprid and then decreased at later stages. The malondialdehyde content significantly increased after 12 h of exposure to imidacloprid compared with the control. Similarly, the enzyme activities of polyphenol oxidase and acetylcholinesterase were increased after the imidacloprid treatment and then decreased at the later stage. In summary, a sublethal dose of imidacloprid caused destructive change in the tissue structure, and this damage was followed by an excessive reactive oxygen species that could not be eliminated by antioxidant enzymes. Our results indicated that the residues of imidacloprid might cause severe toxicity to non-target insects in the environment even far away from the agro-ecosystem where the chemicals were applied.
Keywords: Pyrocoelia analis; antioxidant enzyme activity; imidacloprid; tissue structure; toxicology.
Copyright © 2022 Wang, Cao and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The complete mitochondrial genome of an ornamental firefly Pyrocoelia analis (Coleoptera, Lampyridae).Mitochondrial DNA B Resour. 2022 Jun 1;7(6):911-912. doi: 10.1080/23802359.2022.2078239. eCollection 2022. Mitochondrial DNA B Resour. 2022. PMID: 35692651 Free PMC article.
-
Neurotoxic responses in brain tissues of rainbow trout exposed to imidacloprid pesticide: Assessment of 8-hydroxy-2-deoxyguanosine activity, oxidative stress and acetylcholinesterase activity.Chemosphere. 2017 May;175:186-191. doi: 10.1016/j.chemosphere.2017.02.047. Epub 2017 Feb 11. Chemosphere. 2017. PMID: 28219821
-
Physiological and gene expression responses of Protohermes xanthodes (Megaloptera: Corydalidae) larvae to imidacloprid.Naturwissenschaften. 2024 Sep 9;111(5):46. doi: 10.1007/s00114-024-01932-6. Naturwissenschaften. 2024. PMID: 39249498
-
Insights into the Toxicity and Degradation Mechanisms of Imidacloprid Via Physicochemical and Microbial Approaches.Toxics. 2020 Sep 1;8(3):65. doi: 10.3390/toxics8030065. Toxics. 2020. PMID: 32882955 Free PMC article. Review.
-
Imidacloprid as reproductive toxicant and endocrine disruptor: investigations in laboratory animals.Arh Hig Rada Toksikol. 2018 Jun 1;69(2):103-108. doi: 10.2478/aiht-2018-69-3144. Arh Hig Rada Toksikol. 2018. PMID: 29990292 Review.
Cited by
-
Oral history as a citizen science tool to understand biodiversity loss and environmental changes: on firefly extirpation in Morelia, Michoacán, Mexico.PeerJ. 2025 May 5;13:e19413. doi: 10.7717/peerj.19413. eCollection 2025. PeerJ. 2025. PMID: 40343085 Free PMC article.
-
Illuminating Firefly Diversity: Trends, Threats and Conservation Strategies.Insects. 2024 Jan 19;15(1):71. doi: 10.3390/insects15010071. Insects. 2024. PMID: 38276820 Free PMC article. Review.
-
Multiple Stressor Effects of a Neonicotinoid, Heatwaves, and Elevated Temperatures on Aquatic Insect Emergence.Environ Sci Technol. 2025 Jul 22;59(28):14226-14238. doi: 10.1021/acs.est.5c01498. Epub 2025 Jul 7. Environ Sci Technol. 2025. PMID: 40624829 Free PMC article.
-
Buscando Luciérnagas: findings on Mexican fireflies from an 8-year virtual citizen science project.PeerJ. 2024 Sep 18;12:e18141. doi: 10.7717/peerj.18141. eCollection 2024. PeerJ. 2024. PMID: 39308813 Free PMC article.
-
Physiological response of rapeseed (Brassica napus) to the insecticide imidacloprid.Ecotoxicology. 2025 Jul;34(5):862-875. doi: 10.1007/s10646-025-02883-y. Epub 2025 Apr 19. Ecotoxicology. 2025. PMID: 40252137 Free PMC article.
References
-
- Balieira K. V. B., Mazzo M., Bizerra P. F. V., Guimarães A. R., Nicodemo D., Mingatto F. E. (2018). Imidacloprid-induced Oxidative Stress in Honey Bees and the Antioxidant Action of Caffeine. Apidologie 49, 562–572. 10.1007/s13592-018-0583-1 - DOI
-
- Bálint B., Balogh K., Mézes M., Szabó B. (2021). Differences in the Effects of Sodium Selenate and Sodium Selenite on the Mortality, Reproduction, Lipid Peroxidation and Glutathione Redox Status of Folsomia candida Willem 1902 (Collembola). Eur. J. Soil Biol. 107, 103361. 10.1016/j.ejsobi.2021.103361 - DOI
LinkOut - more resources
Full Text Sources

