Metabolic Regulation of Hematopoietic Stem Cells
- PMID: 35785147
- PMCID: PMC9242402
- DOI: 10.1097/HS9.0000000000000740
Metabolic Regulation of Hematopoietic Stem Cells
Abstract
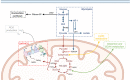
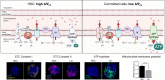
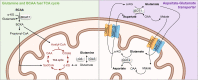
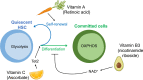
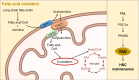
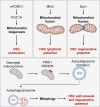
Cellular metabolism is a key regulator of hematopoietic stem cell (HSC) maintenance. HSCs rely on anaerobic glycolysis for energy production to minimize the production of reactive oxygen species and shift toward mitochondrial oxidative phosphorylation upon differentiation. However, increasing evidence has shown that HSCs still maintain a certain level of mitochondrial activity in quiescence, and exhibit high mitochondrial membrane potential, which both support proper HSC function. Since glycolysis and the tricarboxylic acid (TCA) cycle are not directly connected in HSCs, other nutrient pathways, such as amino acid and fatty acid metabolism, generate acetyl-CoA and provide it to the TCA cycle. In this review, we discuss recent insights into the regulatory roles of cellular metabolism in HSCs. Understanding the metabolic requirements of healthy HSCs is of critical importance to the development of new therapies for hematological disorders.
Copyright © 2022 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Figures

References
-
- Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403. - PubMed
-
- Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. - PubMed
-
- Baldwin JE, Krebs H. The evolution of metabolic cycles. Nature. 1981;291:381–382. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
