The Role of Lipid Metabolism in Gastric Cancer
- PMID: 35785165
- PMCID: PMC9240397
- DOI: 10.3389/fonc.2022.916661
The Role of Lipid Metabolism in Gastric Cancer
Abstract
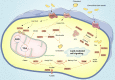
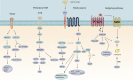
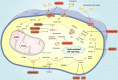
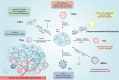
Gastric cancer has been one of the most common cancers worldwide with extensive metastasis and high mortality. Chemotherapy has been found as a main treatment for metastatic gastric cancer, whereas drug resistance limits the effectiveness of chemotherapy and leads to treatment failure. Chemotherapy resistance in gastric cancer has a complex and multifactorial mechanism, among which lipid metabolism plays a vital role. Increased synthesis of new lipids or uptake of exogenous lipids can facilitate the rapid growth of cancer cells and tumor formation. Lipids form the structural basis of biofilms while serving as signal molecules and energy sources. It is noteworthy that lipid metabolism is capable of inducing drug resistance in gastric cancer cells by reshaping the tumor micro-environment. In this study, new mechanisms of lipid metabolism in gastric cancer and the metabolic pathways correlated with chemotherapy resistance are reviewed. In particular, we discuss the effects of lipid metabolism on autophagy, biomarkers treatment and drug resistance in gastric cancer from the perspective of lipid metabolism. In brief, new insights can be gained into the development of promising therapies through an in-depth investigation of the mechanism of lipid metabolism reprogramming and resensitization to chemotherapy in gastric cancer cells, and scientific treatment can be provided by applying lipid-key enzyme inhibitors as cancer chemical sensitizers in clinical settings.
Keywords: biomarkers; chemoresistance; gastric cancer; lipid metabolism; treatment.
Copyright © 2022 Cui, Yi, Zhu and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

