Synthesis, Molecular Modeling Study, and Quantum-Chemical-Based Investigations of Isoindoline-1,3-diones as Antimycobacterial Agents
- PMID: 35785272
- PMCID: PMC9244950
- DOI: 10.1021/acsomega.2c01981
Synthesis, Molecular Modeling Study, and Quantum-Chemical-Based Investigations of Isoindoline-1,3-diones as Antimycobacterial Agents
Abstract
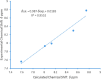
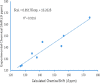
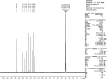
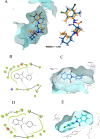
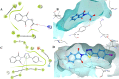
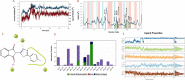
The condensation of phthalic anhydride afforded structurally modified isoindoline-1,3-dione derivatives with selected amino-containing compounds. The title compounds (2-30) have been characterized by thin-layer chromatography (TLC), infrared spectroscopy, 1H and 13C NMR spectroscopy, and mass spectroscopy. All of the compounds were assessed for their antimycobacterial activity toward the H37Rv strain by a dual read-out assay method. Among the synthesized compounds, compound 27 possessed a significant IC50 of 18 μM, making it the most potent compound of the series. The InhA inhibitory (IC50) activity of compound 27 was 8.65 μM in comparison to Triclosan (1.32 μM). Computational studies like density functional theory (DFT) study, molecular docking, and dynamic simulation studies illustrated the reactivity and stability of the synthesized compounds as InhA inhibitors. A quantum-mechanics-based DFT study was carried out to investigate the molecular and electronic properties, reactivities, and nature of bonding present in the synthesized compounds and theoretical vibrational (IR) and isotropic value (1H and 13C NMR) calculations.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



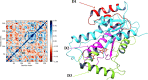
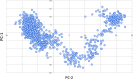
References
-
- World Health Organization. Tuberculosis. https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed 2022-04-01).
LinkOut - more resources
Full Text Sources

