1-Substituted Perylene Derivatives by Anionic Cyclodehydrogenation: Analysis of the Reaction Mechanism
- PMID: 35785287
- PMCID: PMC9245103
- DOI: 10.1021/acsomega.2c02017
1-Substituted Perylene Derivatives by Anionic Cyclodehydrogenation: Analysis of the Reaction Mechanism
Abstract
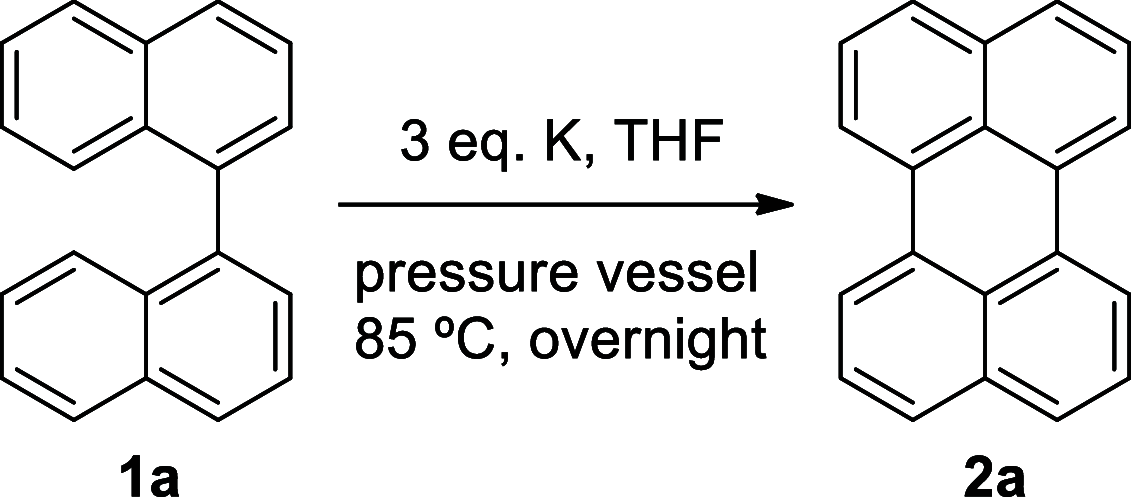
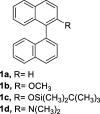
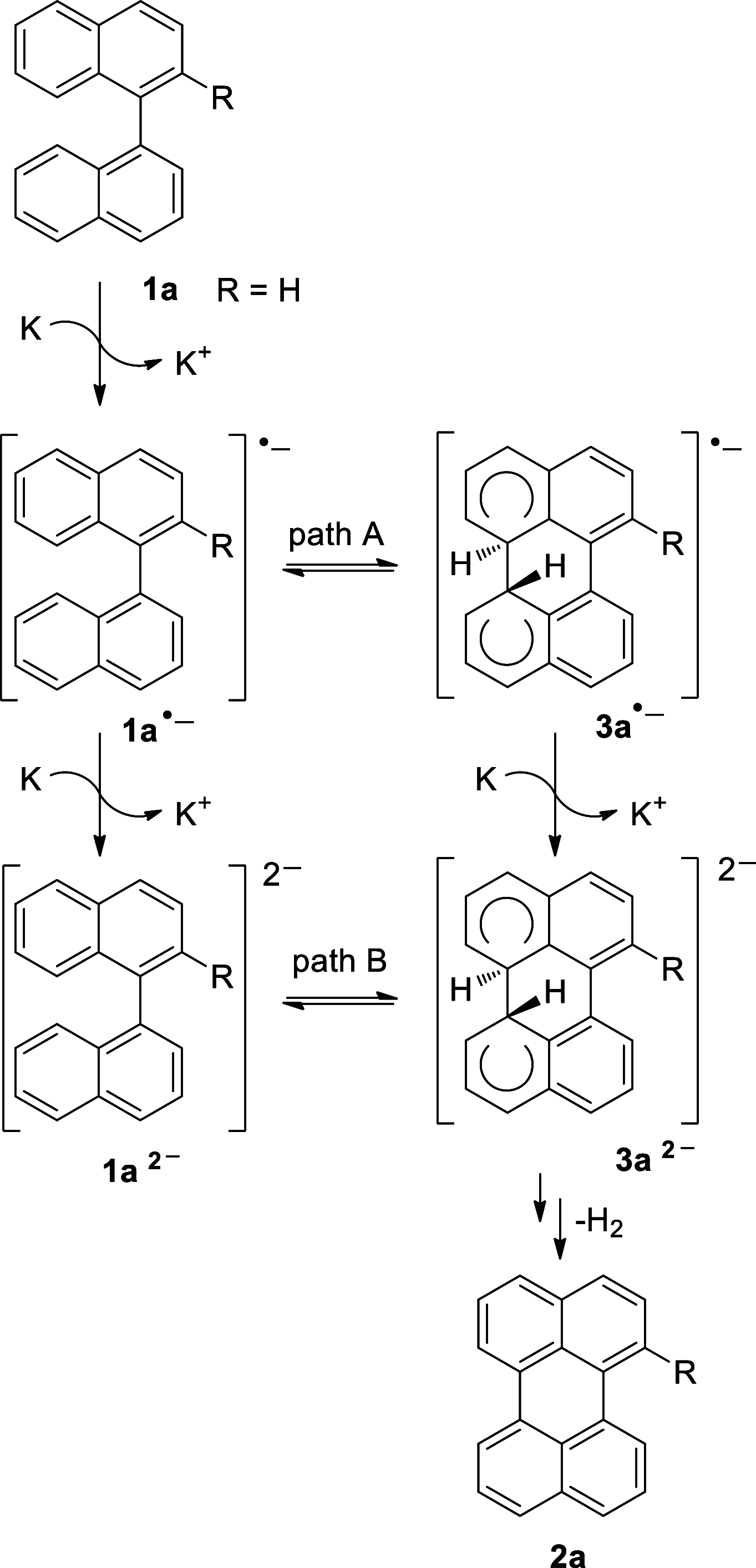
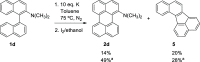
Perylene derivatives constitute a promising class of compounds with technological applications mainly due to their optoelectronic properties. One mechanism proposed to synthesize them, starting from binaphthyl derivatives, is anionic cyclodehydrogenation (under reductive conditions). However, the scope of this reaction is limited. In the present study, we report a theoretical and experimental analysis of this particular reaction mechanism for its use in the synthesis of 1-substituted perylenes. Different substituents at position 2 of 1,1'-binaphthalene were evaluated: -OCH3, -OSi(CH3)2C(CH3)3, and -N(CH3)2. Based on density functional theory (DFT) calculations on the proposed mechanism, we suggest that the cyclization takes place from binaphthyl dianion instead of its radical anion. This dianion has an open-shell diradical nature, and this could be the species that was detected by EPR in previous studies. The O-substituted derivatives could not afford the perylene derivatives since their radical anions fragment and the necessary binaphthyl dianion could not be formed. On the other hand, 49% of N,N-dimethylperylen-1-amine was obtained starting from the N-substituted 2-binapthyl derivative as a substrate, employing a simpler experimental methodology.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Enrichi F.; Righini G. C.. Solar Cells and Light Management: Materials, Strategies and Sustainability; Elsevier: Amsterdam, 2020.
- Yersin H.Highly efficient OLEDs: Materials based on thermally activated delayed fluorescence; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019.
- Li Z. R.Organic light-emitting materials and devices; CRC Press: Boca Raton, 2015.
-
- Kalyani N. T.; Dhoble S. J. Novel materials for fabrication and encapsulation of OLEDs. Renewable Sustainable Energy Rev. 2015, 44, 319–347. 10.1016/j.rser.2014.11.070. - DOI
- Kalyani N. T.; Dhoble S. J. Organic Light Emitting Diodes: energy saving lighting technology-A review. Renewable Sustainable Energy Rev. 2012, 16, 2696–2723. 10.1016/j.rser.2012.02.021. - DOI
-
- Wadsworth A.; Moser M.; Marks A.; Little M. S.; Gasparini N.; Brabec C. J.; Baran D.; McCulloch I. Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem. Soc. Rev. 2019, 48, 1596–1625. 10.1039/c7cs00892a. - DOI - PubMed
- Yan C.; Barlow S.; Wang Z.; Yan H.; Jen A. K. Y.; Marder S. R.; Zhan X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003.10.1038/natrevmats.2018.3. - DOI
-
- Li G.; Yang W.; Wang S.; Liu T.; Yan C.; Li G.; Zhang Y.; Li D.; Wang X.; Hao P.; Li J.; Huo L.; Yan H.; Tang B. Methane-perylene diimide-based small molecule acceptors for high efficiency non-fullerene organic solar cells. J. Mater. Chem. C 2019, 7, 10901–10907. 10.1039/C9TC03457A. - DOI
- Markiewicz J. T.; Wudl F. Perylene, Oligorylenes, and Aza-Analogs. ACS Appl. Mater. Interfaces 2015, 7, 28063–28085. 10.1021/acsami.5b02243. - DOI - PubMed
LinkOut - more resources
Full Text Sources

