Hyperoxia evokes pericyte-mediated capillary constriction
- PMID: 35786054
- PMCID: PMC9580167
- DOI: 10.1177/0271678X221111598
Hyperoxia evokes pericyte-mediated capillary constriction
Abstract
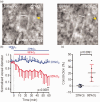
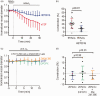
Oxygen supplementation is regularly prescribed to patients to treat or prevent hypoxia. However, excess oxygenation can lead to reduced cerebral blood flow (CBF) in healthy subjects and worsen the neurological outcome of critically ill patients. Most studies on the vascular effects of hyperoxia focus on arteries but there is no research on the effects on cerebral capillary pericytes, which are major regulators of CBF. Here, we used bright-field imaging of cerebral capillaries and modeling of CBF to show that hyperoxia (95% superfused O2) led to an increase in intracellular calcium level in pericytes and a significant capillary constriction, sufficient to cause an estimated 25% decrease in CBF. Although hyperoxia is reported to cause vascular smooth muscle cell contraction via generation of reactive oxygen species (ROS), endothelin-1 and 20-HETE, we found that increased cytosolic and mitochondrial ROS levels and endothelin release were not involved in the pericyte-mediated capillary constriction. However, a 20-HETE synthesis blocker greatly reduced the hyperoxia-evoked capillary constriction. Our findings establish pericytes as regulators of CBF in hyperoxia and 20-HETE synthesis as an oxygen sensor in CBF regulation. The results also provide a mechanism by which clinically administered oxygen can lead to a worse neurological outcome.
Keywords: 20-HETE; Hyperoxia; cerebral blood flow; pericyte; reactive oxygen species.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



Similar articles
-
Amyloid β oligomers constrict human capillaries in Alzheimer's disease via signaling to pericytes.Science. 2019 Jul 19;365(6450):eaav9518. doi: 10.1126/science.aav9518. Epub 2019 Jun 20. Science. 2019. PMID: 31221773 Free PMC article.
-
Active role of capillary pericytes during stimulation-induced activity and spreading depolarization.Brain. 2018 Jul 1;141(7):2032-2046. doi: 10.1093/brain/awy143. Brain. 2018. PMID: 30053174 Free PMC article.
-
Human brain blood flow and metabolism during isocapnic hyperoxia: the role of reactive oxygen species.J Physiol. 2019 Feb;597(3):741-755. doi: 10.1113/JP277122. Epub 2018 Dec 26. J Physiol. 2019. PMID: 30506968 Free PMC article.
-
Pericyte morphology and function.Histol Histopathol. 2021 Jun;36(6):633-643. doi: 10.14670/HH-18-314. Epub 2021 Feb 17. Histol Histopathol. 2021. PMID: 33595091 Review.
-
Pericyte Control of Blood Flow Across Microvascular Zones in the Central Nervous System.Annu Rev Physiol. 2022 Feb 10;84:331-354. doi: 10.1146/annurev-physiol-061121-040127. Epub 2021 Oct 21. Annu Rev Physiol. 2022. PMID: 34672718 Free PMC article. Review.
Cited by
-
Pericytes require physiological oxygen tension to maintain phenotypic fidelity.Sci Rep. 2024 Nov 28;14(1):29581. doi: 10.1038/s41598-024-80682-x. Sci Rep. 2024. PMID: 39609469 Free PMC article.
-
Imaging the time course, morphology, neuronal tissue compression, and resolution of cerebral microhemorrhages in mice using intravital two-photon microscopy: insights into arteriolar, capillary, and venular origin.Geroscience. 2023 Oct;45(5):2851-2872. doi: 10.1007/s11357-023-00839-w. Epub 2023 Jun 20. Geroscience. 2023. PMID: 37338779 Free PMC article.
-
Post-Ischemic Permeability of the Blood-Brain Barrier to Amyloid and Platelets as a Factor in the Maturation of Alzheimer's Disease-Type Brain Neurodegeneration.Int J Mol Sci. 2023 Jun 27;24(13):10739. doi: 10.3390/ijms241310739. Int J Mol Sci. 2023. PMID: 37445917 Free PMC article. Review.
-
Enhanced Cerebral Hemodynamics and Cognitive Function Via Knockout of Dual-Specificity Protein Phosphatase 5.J Pharm Pharmacol Res. 2023;7(2):49-61. doi: 10.26502/fjppr.070. Epub 2023 May 12. J Pharm Pharmacol Res. 2023. PMID: 37588944 Free PMC article.
-
Brain pericyte biology: from physiopathological mechanisms to potential therapeutic applications in ischemic stroke.Front Cell Neurosci. 2023 Sep 14;17:1267785. doi: 10.3389/fncel.2023.1267785. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37780206 Free PMC article. Review.
References
-
- Sjöberg F, Singer M. The medical use of oxygen: a time for critical reappraisal. J Intern Med 2013; 274: 505–528. - PubMed
-
- Maas AI, Fleckenstein W, de Jong DA, et al.. Monitoring cerebral oxygenation: experimental studies and preliminary clinical results of continuous monitoring of cerebrospinal fluid and brain tissue oxygen tension. Acta Neurochir Suppl (Wien) 1993; 59: 50–57. - PubMed
-
- De Georgia MA. Brain tissue oxygen monitoring in neurocritical care. J Intensive Care Med 2015; 30: 473–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

