Efficacy and safety of leflunomide combined with corticosteroids for the treatment of IgA nephropathy: a Meta-analysis of randomized controlled trials
- PMID: 35786300
- PMCID: PMC9262374
- DOI: 10.1080/0886022X.2022.2085576
Efficacy and safety of leflunomide combined with corticosteroids for the treatment of IgA nephropathy: a Meta-analysis of randomized controlled trials
Abstract
Objective: We performed a meta-analysis of randomized controlled trials (RCTs) to evaluate the efficacy and safety of leflunomide combined with corticosteroids, compared with corticosteroids alone, for IgA nephropathy.
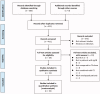
Materials and methods: Studies were retrieved by searching of PubMed, Embase, Cochrane's Library, China National Knowledge Infrastructure (CNKI), and Wanfang databases on 11 October 2021. A random-effect model incorporating the heterogeneity was used to pool the results. The efficacy outcomes included the complete remission rate of proteinuria, overall response rate (the combined rates of patients with complete and partial remission of proteinuria), changes of urine protein excretion (UPE), serum creatinine (SCr), and estimated glomerular infiltrating rate (eGFR).
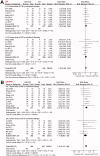
Results: Nineteen studies were included. Patients receiving the combined therapy had a higher complete remission rate (relative risk [RR]: 1.29, 95% CI: 1.08-1.55, p = 0.006; I2 = 0%) and overall response rate (RR: 1.18, 95% CI: 1.10-1.26, p < 0.001, I2 = 0%) compared to patients who received CS alone. Besides, combined therapy was associated with significantly reduced levels of UPE (mean difference [MD]: -0.30 g/24h, 95% CI: -0.43 to -0.16, p < 0.001; I2 = 34%) and SCr (MD: -7.55 mmol/L, 95% CI: -11.06 to -4.04, p < 0.001; I2 = 34%), and increased level of eGFR (MD: 6.51 mL/min/1.73 m2, 95% CI: 4.06-8.97, p < 0.001; I2 = 0%). The incidence of adverse events was not significantly different.
Conclusions: Combined treatment with leflunomide and corticosteroids was more effective than corticosteroids alone for patients with IgA nephropathy.
Keywords: IgA nephropathy; Leflunomide; corticosteroids; meta-analysis; randomized controlled trials.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Suzuki H, Kikuchi M, Koike K, et al. A digest from evidence-based clinical practice guideline for IgA nephropathy 2020. Clin Exp Nephrol. 2021;25(12):1012–1276. - PubMed
-
- Zhang H, Barratt J.. Is IgA nephropathy the same disease in different parts of the world? Semin Immunopathol. 2021;43(5):707–715. - PubMed
-
- Peng XJ, Zheng WM, Fu R, et al. Efficacy and safety of mycophenolate mofetil in the treatment for IgA nephropathy: a meta-analysis of randomized controlled trials. Clin Exp Nephrol. 2021;25(7):788–801. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
