Simultaneous Cellular Imaging, Electrical Recording and Stimulation of Hippocampal Activity in Freely Behaving Mice
- PMID: 35786642
- PMCID: PMC9272116
- DOI: 10.5607/en22011
Simultaneous Cellular Imaging, Electrical Recording and Stimulation of Hippocampal Activity in Freely Behaving Mice
Abstract
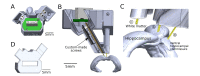
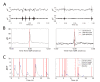
Hippocampal sharp-wave ripple activity (SWRs) and the associated replay of neural activity patterns are well-known for their role in memory consolidation. This activity has been studied using electrophysiological approaches, as high temporal resolution is required to recognize SWRs in the neuronal signals. However, it has been difficult to analyze the individual contribution of neurons to task-specific SWRs, because it is hard to track neurons across a long time with electrophysiological recording. In this study, we recorded local field potential (LFP) signals in the hippocampal CA1 of freely behaving mice and simultaneously imaged calcium signals in contralateral CA1 to leverage the advantages of both electrophysiological and imaging approaches. We manufactured a custom-designed microdrive array and targeted tetrodes to the left hippocampus CA1 for LFP recording and applied electrical stimulation in the ventral hippocampal commissure (VHC) for closed-loop disruption of SWRs. Neuronal population imaging in the right hippocampal CA1 was performed using a miniature fluorescent microscope (Miniscope) and a genetically encoded calcium indicator. As SWRs show highly synchronized bilateral occurrence, calcium signals of SWR-participating neurons could be identified and tracked in spontaneous or SWR-disrupted conditions. Using this approach, we identified a subpopulation of CA1 neurons showing synchronous calcium elevation to SWRs. Our results showed that SWR-related calcium transients are more disrupted by electrical stimulation than non-SWRrelated calcium transients, validating the capability of the system to detect and disrupt SWRs. Our dual recording method can be used to uncover the dynamic participation of individual neurons in SWRs and replay over extended time windows.
Keywords: Brain wave; Calcium; Electrophysiology; Hippocampus.
Figures



References
-
- Kandel ER. In search of memory: The emergence of a new science of mind. W. W. Norton & Company; New York, NY: 2007.
-
- Cai DJ, Aharoni D, Shuman T, Shobe J, Biane J, Song W, Wei B, Veshkini M, La-Vu M, Lou J, Flores SE, Kim I, Sano Y, Zhou M, Baumgaertel K, Lavi A, Kamata M, Tuszynski M, Mayford M, Golshani P, Silva AJ. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 2016;534:115–118. doi: 10.1038/nature17955. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

