Regulatory T Cell Proportion and Phenotype Are Altered in Women Using Oral Contraception
- PMID: 35786711
- PMCID: PMC9354970
- DOI: 10.1210/endocr/bqac098
Regulatory T Cell Proportion and Phenotype Are Altered in Women Using Oral Contraception
Abstract
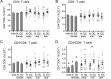
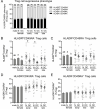
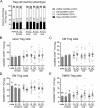
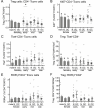
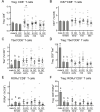
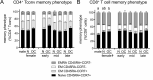
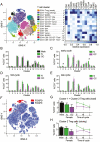
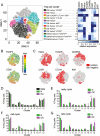
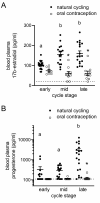
Regulatory T (Treg) cells are a specialized CD4+ T cell subpopulation that are essential for immune homeostasis, immune tolerance, and protection against autoimmunity. There is evidence that sex-steroid hormones estrogen and progesterone modulate Treg cell abundance and phenotype in women. Since natural oscillations in these hormones are modified by hormonal contraceptives, we examined whether oral contraception (OC) use impacts Treg cells and related T cell populations. T cells were analyzed by multiparameter flow cytometry in peripheral blood collected across the menstrual cycle from healthy women either using OC or without hormonal contraception and from age-matched men. Compared to naturally cycling women, women using OC had fewer Treg cells and an altered Treg cell phenotype. Notably, Treg cells exhibiting a strongly suppressive phenotype, defined by high FOXP3, CD25, Helios, HLADR, CTLA4, and Ki67, comprised a lower proportion of total Treg cells, particularly in the early- and mid-cycle phases. The changes were moderate compared to more substantial differences in Treg cells between women and men, wherein women had fewer Treg cells-especially of the effector memory Treg cell subset-associated with more T helper type 1 (Th1) cells and CD8+ T cells and lower Treg:Th1 cell and Treg:CD8+ T cell ratios than men. These findings imply that OC can modulate the number and phenotype of peripheral blood Treg cells and raise the possibility that Treg cells contribute to the physiological changes and altered disease susceptibility linked with OC use.
Keywords: T cells; autoimmunity; regulatory T cells; sex hormones; tolerance.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

Similar articles
-
FOXP3+Helios+ Regulatory T Cells, Immune Activation, and Advancing Disease in HIV-Infected Children.J Acquir Immune Defic Syndr. 2016 Aug 15;72(5):474-84. doi: 10.1097/QAI.0000000000001000. J Acquir Immune Defic Syndr. 2016. PMID: 27003495 Free PMC article.
-
Analysis of CD8+ Treg cells in patients with ovarian cancer: a possible mechanism for immune impairment.Cell Mol Immunol. 2015 Sep;12(5):580-91. doi: 10.1038/cmi.2015.57. Epub 2015 Jul 13. Cell Mol Immunol. 2015. PMID: 26166762 Free PMC article.
-
FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua.Biol Reprod. 2010 Apr;82(4):698-705. doi: 10.1095/biolreprod.109.081208. Epub 2009 Dec 16. Biol Reprod. 2010. PMID: 20018909
-
Genetic and epigenetic basis of Treg cell development and function: from a FoxP3-centered view to an epigenome-defined view of natural Treg cells.Immunol Rev. 2014 May;259(1):192-205. doi: 10.1111/imr.12174. Immunol Rev. 2014. PMID: 24712467 Review.
-
Advances in distinguishing natural from induced Foxp3(+) regulatory T cells.Int J Clin Exp Pathol. 2013;6(2):116-23. Epub 2013 Jan 15. Int J Clin Exp Pathol. 2013. PMID: 23329997 Free PMC article. Review.
Cited by
-
T cells in obesity-associated inflammation: The devil is in the details.Immunol Rev. 2024 Jul;324(1):25-41. doi: 10.1111/imr.13354. Epub 2024 May 20. Immunol Rev. 2024. PMID: 38767210 Free PMC article. Review.
-
Role of Sex and Age in Fatal Outcomes of COVID-19: Women and Older Centenarians Are More Resilient.Int J Mol Sci. 2023 Jan 30;24(3):2638. doi: 10.3390/ijms24032638. Int J Mol Sci. 2023. PMID: 36768959 Free PMC article. Review.
-
Dynamic Changes of Regulatory T Cells/CD4⁺ T Cells in Peripheral Blood of Adult Kidney Transplant Recipients: A Comparison of Pediatric and Adult Kidney Donors.Ann Transplant. 2023 Sep 26;28:e940604. doi: 10.12659/AOT.940604. Ann Transplant. 2023. PMID: 37749867 Free PMC article.
-
Role of cellular prion protein in mouse granulosa cells and its effects on ovarian function in knockout mice.Mol Med Rep. 2025 Oct;32(4):265. doi: 10.3892/mmr.2025.13630. Epub 2025 Jul 25. Mol Med Rep. 2025. PMID: 40709399 Free PMC article.
-
Sex-based differences in the lung immune microenvironment are associated with an increased risk of lung cancer in women.J Thorac Cardiovasc Surg. 2025 Mar;169(3):764-776.e9. doi: 10.1016/j.jtcvs.2024.07.017. Epub 2024 Jul 15. J Thorac Cardiovasc Surg. 2025. PMID: 39019152
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

