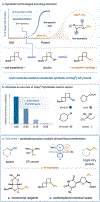
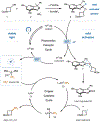
Deoxytrifluoromethylation of Alcohols
- PMID: 35786873
- PMCID: PMC9676087
- DOI: 10.1021/jacs.2c04807
Deoxytrifluoromethylation of Alcohols
Abstract
Deoxy-functionalization of alcohols represents a class of reactions that has had a profound impact on modern medicine. In particular, deoxyfluorination is commonly employed as a means to incorporate high-value fluorine atoms into drug-like molecules. Recently, the trifluoromethyl (CF3) group has garnered attention from medicinal chemists due to its ability to markedly improve the pharmaceutical properties of small-molecule drug candidates. To date, however, there remains no general means to accomplish the analogous deoxygenative trifluoromethylation of alcohols. We report herein a copper metallaphotoredox-mediated direct deoxytrifluoromethylation, wherein alcohol substrates are activated in situ by benzoxazolium salts for C(sp3)-CF3 bond formation.
Conflict of interest statement
The authors declare the following competing financial interest(s): D.W.C.M. declares a competing financial interest with respect to the integrated photoreactor.
Figures
References
-
- Brown DG; Boström J Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone?: Miniperspective. J. Med. Chem 2016, 59 (10), 4443–4458. - PubMed
-
- Walters WP; Green J; Weiss JR; Murcko MA What Do Medicinal Chemists Actually Make? A 50-Year Retrospective. J. Med. Chem 2011, 54 (19), 6405–6416. - PubMed
-
- Wang Y; Haight I; Gupta R; Vasudevan A What Is in Our Kit? An Analysis of Building Blocks Used in Medicinal Chemistry Parallel Libraries. J. Med. Chem 2021, 64 (23), 17115–17122. - PubMed
-
- Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52 (21), 6752–6756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources