Glioblastoma: Current Status, Emerging Targets, and Recent Advances
- PMID: 35786935
- PMCID: PMC9297300
- DOI: 10.1021/acs.jmedchem.1c01946
Glioblastoma: Current Status, Emerging Targets, and Recent Advances
Abstract
Glioblastoma (GBM) is a highly malignant brain tumor characterized by a heterogeneous population of genetically unstable and highly infiltrative cells that are resistant to chemotherapy. Although substantial efforts have been invested in the field of anti-GBM drug discovery in the past decade, success has primarily been confined to the preclinical level, and clinical studies have often been hampered due to efficacy-, selectivity-, or physicochemical property-related issues. Thus, expansion of the list of molecular targets coupled with a pragmatic design of new small-molecule inhibitors with central nervous system (CNS)-penetrating ability is required to steer the wheels of anti-GBM drug discovery endeavors. This Perspective presents various aspects of drug discovery (challenges in GBM drug discovery and delivery, therapeutic targets, and agents under clinical investigation). The comprehensively covered sections include the recent medicinal chemistry campaigns embarked upon to validate the potential of numerous enzymes/proteins/receptors as therapeutic targets in GBM.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
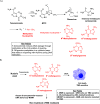

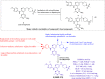
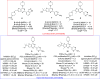


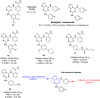



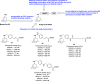
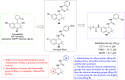
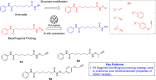
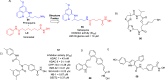



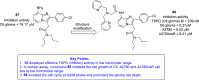
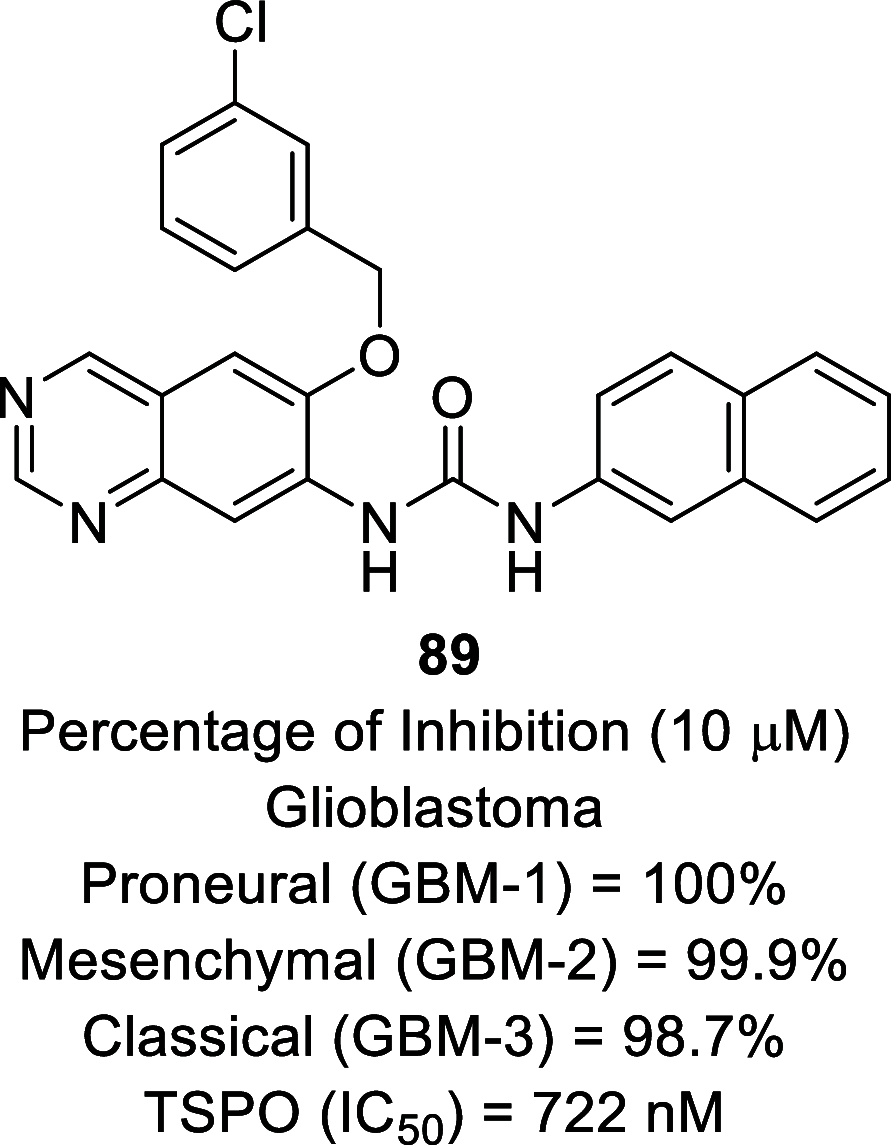
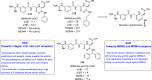
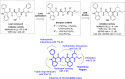
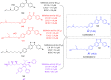
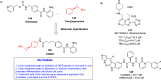

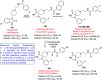
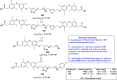
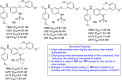
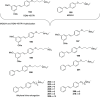
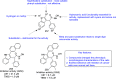

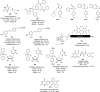
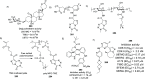

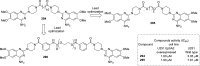
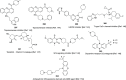
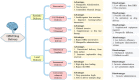
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Miscellaneous

