A long noncoding RNA influences the choice of the X chromosome to be inactivated
- PMID: 35787055
- PMCID: PMC9282422
- DOI: 10.1073/pnas.2118182119
A long noncoding RNA influences the choice of the X chromosome to be inactivated
Abstract
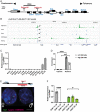
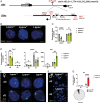
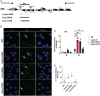
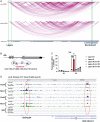
X chromosome inactivation (XCI) is the process of silencing one of the X chromosomes in cells of the female mammal which ensures dosage compensation between the sexes. Although theoretically random in somatic tissues, the choice of which X chromosome is chosen to be inactivated can be biased in mice by genetic element(s) associated with the so-called X-controlling element (Xce). Although the Xce was first described and genetically localized nearly 40 y ago, its mode of action remains elusive. In the approach presented here, we identify a single long noncoding RNA (lncRNA) within the Xce locus, Lppnx, which may be the driving factor in the choice of which X chromosome will be inactivated in the developing female mouse embryo. Comparing weak and strong Xce alleles we show that Lppnx modulates the expression of Xist lncRNA, one of the key factors in XCI, by controlling the occupancy of pluripotency factors at Intron1 of Xist. This effect is counteracted by enhanced binding of Rex1 in DxPas34, another key element in XCI regulating the activity of Tsix lncRNA, the main antagonist of Xist, in the strong but not in the weak Xce allele. These results suggest that the different susceptibility for XCI observed in weak and strong Xce alleles results from differential transcription factor binding of Xist Intron 1 and DxPas34, and that Lppnx represents a decisive factor in explaining the action of the Xce.
Keywords: X chromosome inactivation; X-controlling element; female mouse embryo; noncoding RNA; pluripotency factors.
Conflict of interest statement
The authors declare no competing interest.
Figures

Comment in
-
Lppnx lncRNA: The new kid on the block or an old friend in X-inactivation choice?Proc Natl Acad Sci U S A. 2023 Feb 14;120(7):e2218989120. doi: 10.1073/pnas.2218989120. Epub 2023 Feb 7. Proc Natl Acad Sci U S A. 2023. PMID: 36749727 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

