STING activation promotes robust immune response and NK cell-mediated tumor regression in glioblastoma models
- PMID: 35787058
- PMCID: PMC9282249
- DOI: 10.1073/pnas.2111003119
STING activation promotes robust immune response and NK cell-mediated tumor regression in glioblastoma models
Abstract
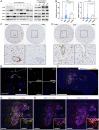
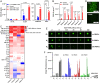
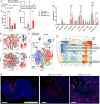
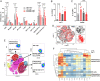
Immunotherapy has had a tremendous impact on cancer treatment in the past decade, with hitherto unseen responses at advanced and metastatic stages of the disease. However, the aggressive brain tumor glioblastoma (GBM) is highly immunosuppressive and remains largely refractory to current immunotherapeutic approaches. The stimulator of interferon genes (STING) DNA sensing pathway has emerged as a next-generation immunotherapy target with potent local immune stimulatory properties. Here, we investigated the status of the STING pathway in GBM and the modulation of the brain tumor microenvironment (TME) with the STING agonist ADU-S100. Our data reveal the presence of STING in human GBM specimens, where it stains strongly in the tumor vasculature. We show that human GBM explants can respond to STING agonist treatment by secretion of inflammatory cytokines. In murine GBM models, we show a profound shift in the tumor immune landscape after STING agonist treatment, with massive infiltration of the tumor-bearing hemisphere with innate immune cells including inflammatory macrophages, neutrophils, and natural killer (NK) populations. Treatment of established murine intracranial GL261 and CT-2A tumors by biodegradable ADU-S100-loaded intracranial implants demonstrated a significant increase in survival in both models and long-term survival with immune memory in GL261. Responses to treatment were abolished by NK cell depletion. This study reveals therapeutic potential and deep remodeling of the TME by STING activation in GBM and warrants further examination of STING agonists alone or in combination with other immunotherapies such as cancer vaccines, chimeric antigen receptor T cells, NK therapies, and immune checkpoint blockade.
Keywords: NK cells; STING; glioblastoma; immunotherapy.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Sharma P., Allison J. P., The future of immune checkpoint therapy. Science 348, 56–61 (2015). - PubMed
-
- Schumacher T. N., Schreiber R. D., Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015). - PubMed
-
- Stupp R., et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group, Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

