The Effect of Tailored, Daily, Smartphone Feedback to Lifestyle Self-Monitoring on Weight Loss at 12 Months: the SMARTER Randomized Clinical Trial
- PMID: 35787516
- PMCID: PMC9297147
- DOI: 10.2196/38243
The Effect of Tailored, Daily, Smartphone Feedback to Lifestyle Self-Monitoring on Weight Loss at 12 Months: the SMARTER Randomized Clinical Trial
Abstract
Background: Self-monitoring (SM) is the centerpiece of behavioral weight loss treatment, but the efficacy of smartphone-delivered SM feedback (FB) has not been tested in large, long-term, randomized trials.
Objective: The aim of this study was to establish the efficacy of providing remote FB to diet, physical activity (PA), and weight SM on improving weight loss outcomes when comparing the SM plus FB (SM+FB) condition to the SM-only condition in a 12-month randomized controlled trial. The study was a single-site, population-based trial that took place in southwestern Pennsylvania, USA, conducted between 2018 and 2021. Participants were smartphone users age ≥18 years, able to engage in moderate PA, with a mean BMI between 27 and 43 kg/m2.
Methods: All participants received a 90-minute, one-to-one, in-person behavioral weight loss counseling session addressing behavioral strategies, establishing participants' dietary and PA goals, and instructing on use of the PA tracker (Fitbit Charge 2), smart scale, and diet SM app. Only SM+FB participants had access to an investigator-developed smartphone app that read SM data, in which an algorithm selected tailored messages sent to the smartphone up to 3 times daily. The SM-only participants did not receive any tailored FB based on SM data. The primary outcome was percent weight change from baseline to 12 months. Secondary outcomes included engagement with digital tools (eg, monthly percentage of FB messages opened and monthly percentage of days adherent to the calorie goal).
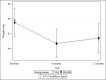
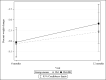
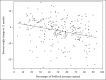
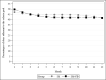
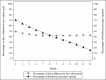
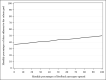
Results: Participants (N=502) were on average 45.0 (SD 14.4) years old with a mean BMI of 33.7 (SD 4.0) kg/m2. The sample was 79.5% female (n=399/502) and 82.5% White (n=414/502). At 12 months, retention was 78.5% (n=394/502) and similar by group (SM+FB: 202/251, 80.5%; SM: 192/251, 76.5%; P=.28). There was significant percent weight loss from baseline in both groups (SM+FB: -2.12%, 95% CI -3.04% to -1.21%, P<.001; SM: -2.39%, 95% CI -3.32% to -1.47%; P<.001), but no difference between the groups (-0.27%; 95% CI -1.57% to 1.03%; t =-0.41; P=.68). Similarly, 26.3% (66/251) of the SM+FB group and 29.1% (73/251) of the SM group achieved ≥5% weight loss (chi-square value=0.49; P=.49). A 1% increase in FB messages opened was associated with a 0.10 greater percent weight loss at 12 months (b=-0.10; 95% CI -0.13 to -0.07; t =-5.90; P<.001). A 1% increase in FB messages opened was associated with 0.12 greater percentage of days adherent to the calorie goal per month (b=0.12; 95% CI 0.07-0.17; F=22.19; P<.001).
Conclusions: There were no significant between-group differences in weight loss; however, the findings suggested that the use of commercially available digital SM tools with or without FB resulted in a clinically significant weight loss in over 25% of participants. Future studies need to test additional strategies that will promote greater engagement with digital tools.
Trial registration: Clinicaltrials.gov NCT03367936; https://clinicaltrials.gov/ct2/show/NCT03367936.
Keywords: adherence; behavioral intervention/weight loss; digital health; engagement; feedback messages; mHealth; obesity; physical activity trackers; randomized clinical trial; self-monitoring; smart scales.
©Lora E Burke, Susan M Sereika, Zhadyra Bizhanova, Bambang Parmanto, Jacob Kariuki, Jessica Cheng, Britney Beatrice, Maribel Cedillo, I Wayan Pulantara, Yuhan Wang, India Loar, Molly B Conroy. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 05.07.2022.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen W, Smith SC, Tomaselli GF, American COCHATFOPG, Obesity S. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014 Jun 24;129(25 Suppl 2):S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=24222017 01.cir.0000437739.71477.ee - DOI - PMC - PubMed
-
- LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O'Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the us preventive services task force. JAMA. 2018 Sep 18;320(11):1172–1191. doi: 10.1001/jama.2018.7777.2702877 - DOI - PubMed
-
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020 Feb;(360):1–8. http://www.cdc.gov/nchs/data/databriefs/db360-h.pdf - PubMed
-
- Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011 Dec;34(4):841–59. doi: 10.1016/j.psc.2011.08.006. http://europepmc.org/abstract/MED/22098808 S0193-953X(11)00083-9 - DOI - PMC - PubMed

