The use of viral vectors in vaccine development
- PMID: 35787629
- PMCID: PMC9253346
- DOI: 10.1038/s41541-022-00503-y
The use of viral vectors in vaccine development
Abstract
Vaccines represent the single most cost-efficient and equitable way to combat and eradicate infectious diseases. While traditional licensed vaccines consist of either inactivated/attenuated versions of the entire pathogen or subunits of it, most novel experimental vaccines against emerging infectious diseases employ nucleic acids to produce the antigen of interest directly in vivo. These include DNA plasmid vaccines, mRNA vaccines, and recombinant viral vectors. The advantages of using nucleic acid vaccines include their ability to induce durable immune responses, high vaccine stability, and ease of large-scale manufacturing. In this review, we present an overview of pre-clinical and clinical data on recombinant viral vector vaccines and discuss the advantages and limitations of the different viral vector platforms.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
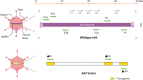
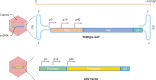

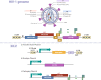

References
-
- Jackson DA, Symons RH, Berg P. Biochemical method for inserting new genetic information into DNA of Simian Virus 40: circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc. Natl Acad. Sci. USA. 1972;69:2904–2909. doi: 10.1073/pnas.69.10.2904. - DOI - PMC - PubMed
-
- Henao-Restrepo AM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) Lancet. 2017;389:505–518. doi: 10.1016/S0140-6736(16)32621-6. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

