Sex differences in heart mitochondria regulate diastolic dysfunction
- PMID: 35787630
- PMCID: PMC9253085
- DOI: 10.1038/s41467-022-31544-5
Sex differences in heart mitochondria regulate diastolic dysfunction
Abstract
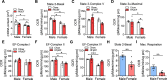
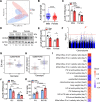
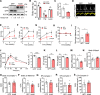
Heart failure with preserved ejection fraction (HFpEF) exhibits a sex bias, being more common in women than men, and we hypothesize that mitochondrial sex differences might underlie this bias. As part of genetic studies of heart failure in mice, we observe that heart mitochondrial DNA levels and function tend to be reduced in females as compared to males. We also observe that expression of genes encoding mitochondrial proteins are higher in males than females in human cohorts. We test our hypothesis in a panel of genetically diverse inbred strains of mice, termed the Hybrid Mouse Diversity Panel (HMDP). Indeed, we find that mitochondrial gene expression is highly correlated with diastolic function, a key trait in HFpEF. Consistent with this, studies of a "two-hit" mouse model of HFpEF confirm that mitochondrial function differs between sexes and is strongly associated with a number of HFpEF traits. By integrating data from human heart failure and the mouse HMDP cohort, we identify the mitochondrial gene Acsl6 as a genetic determinant of diastolic function. We validate its role in HFpEF using adenoviral over-expression in the heart. We conclude that sex differences in mitochondrial function underlie, in part, the sex bias in diastolic function.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
Unexplored horizons on sex bias and progression of heart failure with preserved ejection fraction.Eur Heart J Cardiovasc Pharmacother. 2023 Sep 20;9(6):502-504. doi: 10.1093/ehjcvp/pvad050. Eur Heart J Cardiovasc Pharmacother. 2023. PMID: 37486244 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

