Z-DNA is remodelled by ZBTB43 in prospermatogonia to safeguard the germline genome and epigenome
- PMID: 35787683
- PMCID: PMC9276527
- DOI: 10.1038/s41556-022-00941-9
Z-DNA is remodelled by ZBTB43 in prospermatogonia to safeguard the germline genome and epigenome
Abstract
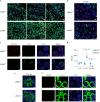
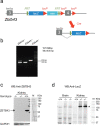
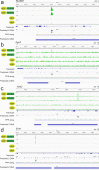
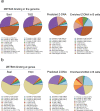
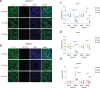
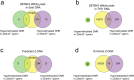
Mutagenic purine-pyrimidine repeats can adopt the left-handed Z-DNA conformation. DNA breaks at potential Z-DNA sites can lead to somatic mutations in cancer or to germline mutations that are transmitted to the next generation. It is not known whether any mechanism exists in the germ line to control Z-DNA structure and DNA breaks at purine-pyrimidine repeats. Here we provide genetic, epigenomic and biochemical evidence for the existence of a biological process that erases Z-DNA specifically in germ cells of the mouse male foetus. We show that a previously uncharacterized zinc finger protein, ZBTB43, binds to and removes Z-DNA, preventing the formation of DNA double-strand breaks. By removing Z-DNA, ZBTB43 also promotes de novo DNA methylation at CG-containing purine-pyrimidine repeats in prospermatogonia. Therefore, the genomic and epigenomic integrity of the species is safeguarded by remodelling DNA structure in the mammalian germ line during a critical window of germline epigenome reprogramming.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

