Anticoagulants for thrombosis prophylaxis in acutely ill patients admitted to hospital: systematic review and network meta-analysis
- PMID: 35788047
- PMCID: PMC9251634
- DOI: 10.1136/bmj-2022-070022
Anticoagulants for thrombosis prophylaxis in acutely ill patients admitted to hospital: systematic review and network meta-analysis
Abstract
Objective: To assess the benefits and harms of different types and doses of anticoagulant drugs for the prevention of venous thromboembolism in patients who are acutely ill and admitted to hospital.
Design: Systematic review and network meta-analysis.
Data sources: Cochrane CENTRAL, PubMed/Medline, Embase, Web of Science, clinical trial registries, and national health authority databases. The search was last updated on 16 November 2021.
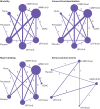
Eligibility criteria for selecting studies: Published and unpublished randomised controlled trials that evaluated low or intermediate dose low-molecular-weight heparin, low or intermediate dose unfractionated heparin, direct oral anticoagulants, pentasaccharides, placebo, or no intervention for the prevention of venous thromboembolism in acutely ill adult patients in hospital.
Main outcome measures: Random effects, bayesian network meta-analyses used four co-primary outcomes: all cause mortality, symptomatic venous thromboembolism, major bleeding, and serious adverse events at or closest timing to 90 days. Risk of bias was also assessed using the Cochrane risk-of-bias 2.0 tool. The quality of evidence was graded using the Confidence in Network Meta-Analysis framework.
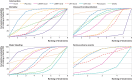
Results: 44 randomised controlled trials that randomly assigned 90 095 participants were included in the main analysis. Evidence of low to moderate quality suggested none of the interventions reduced all cause mortality compared with placebo. Pentasaccharides (odds ratio 0.32, 95% credible interval 0.08 to 1.07), intermediate dose low-molecular-weight heparin (0.66, 0.46 to 0.93), direct oral anticoagulants (0.68, 0.33 to 1.34), and intermediate dose unfractionated heparin (0.71, 0.43 to 1.19) were most likely to reduce symptomatic venous thromboembolism (very low to low quality evidence). Intermediate dose unfractionated heparin (2.63, 1.00 to 6.21) and direct oral anticoagulants (2.31, 0.82 to 6.47) were most likely to increase major bleeding (low to moderate quality evidence). No conclusive differences were noted between interventions regarding serious adverse events (very low to low quality evidence). When compared with no intervention instead of placebo, all active interventions did more favourably with regard to risk of venous thromboembolism and mortality, and less favourably with regard to risk of major bleeding. The results were robust in prespecified sensitivity and subgroup analyses.
Conclusions: Low-molecular-weight heparin in an intermediate dose appears to confer the best balance of benefits and harms for prevention of venous thromboembolism. Unfractionated heparin, in particular the intermediate dose, and direct oral anticoagulants had the least favourable profile. A systematic discrepancy was noted in intervention effects that depended on whether placebo or no intervention was the reference treatment. Main limitations of this study include the quality of the evidence, which was generally low to moderate due to imprecision and within-study bias, and statistical inconsistency, which was addressed post hoc.
Systematic review registration: PROSPERO CRD42020173088.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: RE declares a personal grant from the Groninger AGIKO programme, funded by the University Medical Center Groningen, no support from any organisation for the submitted work; AJS has been a paid consultant by Janssen-Cilag and GlaxoSmithKline. KM reports consulting fees for discussion of gene therapy study results in haemophilia B (fees paid to institution); speaker fees for presentations on haemophilia treatment and DOAC antidotes (fees paid to institution); steering committee for trial of factor VIII concentrate for haemophilia for Bayer; data safety and monitoring board for trial of prothrombin complex concentrate for Octapharma (fees paid to institution); the other authors declare no competing interests.
Figures



Comment in
-
In acutely ill inpatients, benefits and harms of anticoagulants for VTE prophylaxis were assessed.Ann Intern Med. 2022 Nov;175(11):JC128. doi: 10.7326/J22-0089. Epub 2022 Nov 1. Ann Intern Med. 2022. PMID: 36315952
References
-
- National Institute for Health and Care Excellence. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. Published Online First: 2018.https://www.nice.org.uk/guidance/ng89 - PubMed
-
- Food and Drug Administration (United States). Drug approval reports. www.accessdata.fda.gov/scripts/cder/daf/ (accessed 7 Feb 2019).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
