Is Severity Score Associated With Indication for Hematopoietic Stem Cell Transplantation in Individuals With Sickle Cell Anemia?
- PMID: 35788087
- PMCID: PMC10979754
- DOI: 10.1016/j.jtct.2022.06.024
Is Severity Score Associated With Indication for Hematopoietic Stem Cell Transplantation in Individuals With Sickle Cell Anemia?
Abstract
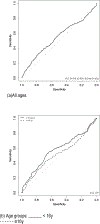
Manifestations of sickle cell disease (SCD) begin early in childhood and cause morbidity and decreased life expectancy. Hematopoietic stem cell transplantation (HSCT) is curative but associated with risk of mortality attributable to the transplant. This risk should be counterbalanced with SCD morbidity and mortality. A severity score using a Bayesian network model was previously validated to predict the risk of death in adult individuals with SCD. The objective of this study is to calculate the severity scores of participants in a multicenter cohort of Brazilians with SCD, using a previously published Bayesian network-derived score, associated with risk of death and then compare the severity scores between participants with and without an indication for HSCT as defined by the Brazilian Ministry of Health (MoH) criteria. This is an observational, retrospective study. We analyzed 2063 individuals with sickle cell anemia from the Recipient Epidemiology and Donor Evaluation Study-III Brazil SCD cohort and applied a Bayesian network-derived score to compare candidates and non-candidates for HSCT according to the Brazilian MoH transplant criteria. Classical statistical methods were used to analyze data and make comparisons. We compared severity scores between cohort members with (n = 431) and without (n = 1632) HSCT indications according to Brazilian MoH. Scores were not different in adult participants with ≥1 HSCT indication when compared to those with no indication (mean 0.342 versus 0.292; median 0.194 versus 0.183, P = .354) and receiver operating characteristic curves did not demonstrate an obvious threshold to differentiate participants with or without HSCT indications. Severity score may predict risk of death but does not differentiate HSCT candidates. Current indications should be evaluated to ensure that patients with more severe disease who might benefit from HSCT are appropriately identified.
Keywords: Bayesian model; Candidates; Cohort; HSCT; Hematopoietic stem cell transplantation; Severity score; Sickle cell anemia; Sickle cell disease.
Copyright © 2022 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement There are no conflicts of interest to report.
Figures


References
-
- Organization WH. Fifty-ninth World Health Assembly: resolutions and decisions, annexes Geneva: World Health Organization; 2006.
-
- Piel FB, Steinberg MH, Rees DC. Sickle Cell Disease. N Engl J Med 2017;377(3):305. - PubMed
-
- Houwing ME, de Pagter PJ, van Beers EJ, Biemond BJ, Rettenbacher E, Rijneveld AW, et al. Sickle cell disease: Clinical presentation and management of a global health challenge. Blood Rev 2019;37:100580. - PubMed

