Differential Effects of the G-Protein-Coupled Estrogen Receptor (GPER) on Rat Embryonic (E18) Hippocampal and Cortical Neurons
- PMID: 35788105
- PMCID: PMC9291730
- DOI: 10.1523/ENEURO.0475-21.2022
Differential Effects of the G-Protein-Coupled Estrogen Receptor (GPER) on Rat Embryonic (E18) Hippocampal and Cortical Neurons
Abstract
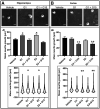
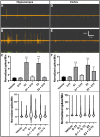
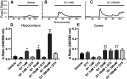
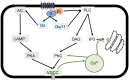
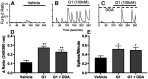
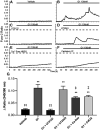
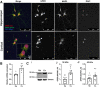
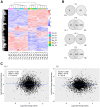
Estrogen plays fundamental roles in nervous system development and function. Traditional studies examining the effect of estrogen in the brain have focused on the nuclear estrogen receptors (ERs), ERα and ERβ. Studies related to the extranuclear, membrane-bound G-protein-coupled ER (GPER/GPR30) have revealed a neuroprotective role for GPER in mature neurons. In this study, we investigated the differential effects of GPER activation in primary rat embryonic day 18 (E18) hippocampal and cortical neurons. Microscopy imaging, multielectrode array (MEA), and Ca2+ imaging experiments revealed that GPER activation with selective agonist, G-1, and nonselective agonist, 17β-estradiol (E2), increased neural growth, neural firing activity, and intracellular Ca2+ more profoundly in hippocampal neurons than in cortical neurons. The GPER-mediated Ca2+ rise in hippocampal neurons involves internal Ca2+ store release via activation of phospholipase C (PLC) and extracellular entry via Ca2+ channels. Immunocytochemistry results revealed no observable difference in GPER expression/localization in neurons, yet real-time qPCR (RT-qPCR) and Western blotting showed a higher GPER expression in the cortex than hippocampus, implying that GPER expression level may not fully account for its robust physiological effects in hippocampal neurons. We used RNA sequencing data to identify distinctly enriched pathways and significantly expressed genes in response to G-1 or E2 in cultured rat E18 hippocampal and cortical neurons. In summary, the identification of differential effects of GPER activation on hippocampal and cortical neurons in the brain and the determination of key genes and molecular pathways are instrumental toward an understanding of estrogen's action in early neuronal development.
Keywords: G-protein-coupled estrogen receptor (GPER/GPR30); electrophysiology; estrogen; hippocampus; neurodevelopment; transcriptome.
Copyright © 2022 Pemberton et al.
Figures

Similar articles
-
Novel GPER Agonist, CITFA, Increases Neurite Growth in Rat Embryonic (E18) Hippocampal Neurons.ACS Chem Neurosci. 2022 Apr 20;13(8):1119-1128. doi: 10.1021/acschemneuro.1c00811. Epub 2022 Mar 30. ACS Chem Neurosci. 2022. PMID: 35353510
-
17β-Estradiol and Agonism of G-protein-Coupled Estrogen Receptor Enhance Hippocampal Memory via Different Cell-Signaling Mechanisms.J Neurosci. 2016 Mar 16;36(11):3309-21. doi: 10.1523/JNEUROSCI.0257-15.2016. J Neurosci. 2016. PMID: 26985039 Free PMC article.
-
Estrogen receptor α and G-protein coupled receptor 30 mediate the neuroprotective effects of 17β-estradiol in novel murine hippocampal cell models.Neuroscience. 2010 Sep 29;170(1):54-66. doi: 10.1016/j.neuroscience.2010.06.076. Epub 2010 Jul 7. Neuroscience. 2010. PMID: 20619320
-
Role of G-protein-coupled estrogen receptor (GPER/GPR30) in maintenance of meiotic arrest in fish oocytes.J Steroid Biochem Mol Biol. 2017 Mar;167:153-161. doi: 10.1016/j.jsbmb.2016.12.005. Epub 2016 Dec 19. J Steroid Biochem Mol Biol. 2017. PMID: 28007532 Review.
-
Estrogen biology: new insights into GPER function and clinical opportunities.Mol Cell Endocrinol. 2014 May 25;389(1-2):71-83. doi: 10.1016/j.mce.2014.02.002. Epub 2014 Feb 12. Mol Cell Endocrinol. 2014. PMID: 24530924 Free PMC article. Review.
Cited by
-
Zebrafish as an emerging model to study estrogen receptors in neural development.Front Endocrinol (Lausanne). 2023 Aug 17;14:1240018. doi: 10.3389/fendo.2023.1240018. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37664862 Free PMC article. Review.
-
Selective Activation of G Protein-Coupled Estrogen Receptor 1 (GPER1) Reduces ER Stress and Pyroptosis via AMPK Signaling Pathway in Experimental Subarachnoid Hemorrhage.Mol Neurobiol. 2025 Jan;62(1):871-884. doi: 10.1007/s12035-024-04312-3. Epub 2024 Jun 27. Mol Neurobiol. 2025. PMID: 38935231
-
Commentary: harnessing the first peptidic modulator of the estrogen receptor GPER.Front Pharmacol. 2024 May 1;15:1413058. doi: 10.3389/fphar.2024.1413058. eCollection 2024. Front Pharmacol. 2024. PMID: 38751778 Free PMC article. No abstract available.
-
G-protein coupled estrogen receptor 1, amyloid-β, and tau tangles in older adults.Commun Biol. 2024 May 15;7(1):569. doi: 10.1038/s42003-024-06272-9. Commun Biol. 2024. PMID: 38750228 Free PMC article.
-
Kif21B mediates the effect of estradiol on the morphological plasticity of mouse hippocampal neurons.Front Mol Neurosci. 2023 Apr 3;16:1143024. doi: 10.3389/fnmol.2023.1143024. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37078090 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
