Design principles for water dissociation catalysts in high-performance bipolar membranes
- PMID: 35788131
- PMCID: PMC9253156
- DOI: 10.1038/s41467-022-31429-7
Design principles for water dissociation catalysts in high-performance bipolar membranes
Abstract
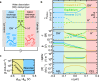
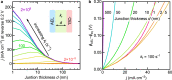
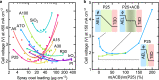
Water dissociation (WD, H2O → H+ + OH-) is the core process in bipolar membranes (BPMs) that limits energy efficiency. Both electric-field and catalytic effects have been invoked to describe WD, but the interplay of the two and the underlying design principles for WD catalysts remain unclear. Using precise layers of metal-oxide nanoparticles, membrane-electrolyzer platforms, materials characterization, and impedance analysis, we illustrate the role of electronic conductivity in modulating the performance of WD catalysts in the BPM junction through screening and focusing the interfacial electric field and thus electrochemical potential gradients. In contrast, the ionic conductivity of the same layer is not a significant factor in limiting performance. BPM water electrolyzers, optimized via these findings, use ~30-nm-diameter anatase TiO2 as an earth-abundant WD catalyst, and generate O2 and H2 at 500 mA cm-2 with a record-low total cell voltage below 2 V. These advanced BPMs might accelerate deployment of new electrodialysis, carbon-capture, and carbon-utilization technology.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Giesbrecht PK, Freund MS. Recent advances in bipolar membrane design and applications. Chem. Mater. 2020;32:8060–8090. doi: 10.1021/acs.chemmater.0c02829. - DOI
-
- Pärnamäe R, et al. Bipolar membranes: a review on principles, latest developments, and applications. J. Membr. Sci. 2021;617:118538. doi: 10.1016/j.memsci.2020.118538. - DOI
-
- Fuller CS. Some analogies between semiconductors and electrolyte solutions. Rec. Chem. Prog. 1956;17:75–93.
-
- Frilette VJ. Preparation and characterization of bipolar ion exchange membranes. J. Phys. Chem. 1956;60:435–439. doi: 10.1021/j150538a013. - DOI
-
- Lovrecek B, Despic A, Bockris JOM. Electrolytic junctions with rectifying properties. J. Phys. Chem. 1959;63:750–751. doi: 10.1021/j150575a030. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

