Structural basis for inhibition and regulation of a chitin synthase from Candida albicans
- PMID: 35788183
- PMCID: PMC9359617
- DOI: 10.1038/s41594-022-00791-x
Structural basis for inhibition and regulation of a chitin synthase from Candida albicans
Abstract
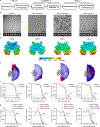
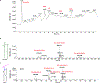
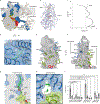
Chitin is an essential component of the fungal cell wall. Chitin synthases (Chss) catalyze chitin formation and translocation across the membrane and are targets of antifungal agents, including nikkomycin Z and polyoxin D. Lack of structural insights into the action of these inhibitors on Chs has hampered their further development to the clinic. We present the cryo-EM structures of Chs2 from Candida albicans (CaChs2) in the apo, substrate-bound, nikkomycin Z-bound, and polyoxin D-bound states. CaChs2 adopts a unique domain-swapped dimer configuration where a conserved motif in the domain-swapped region controls enzyme activity. CaChs2 has a dual regulation mechanism where the chitin translocation tunnel is closed by the extracellular gate and plugged by a lipid molecule in the apo state to prevent non-specific leak. Analyses of substrate and inhibitor binding provide insights into the chemical logic of Chs inhibition, which can guide Chs-targeted antifungal development.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures




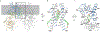




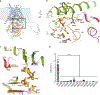
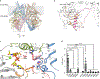

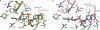

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

