COVID-19 vaccine hesitancy and acceptance: a comprehensive scoping review of global literature
- PMID: 35788306
- PMCID: PMC9278223
- DOI: 10.1093/heapro/daac078
COVID-19 vaccine hesitancy and acceptance: a comprehensive scoping review of global literature
Abstract
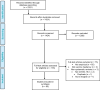
As countries continue the third year of the pandemic, we believe that there has been unfair attention to COVID-19 vaccine efficacy and safety, while tacitly ignoring serious challenges with vaccine uptake, without which vaccination may not be effective against the spread of COVID-19. While several studies have been published on COVID-19 vaccine hesitancy, there remains a need to conduct a comprehensive global analysis of vaccine hesitancy. We conducted a scoping review of 60 studies published globally on vaccine hesitancy and acceptance. We conducted a qualitative analysis to identify motivators and barriers to vaccination across several cultural and demographic contexts. We found the following factors to be relevant in any discussion about addressing or minimizing vaccine hesitancy: risk perceptions, trust in health care systems, solidarity, previous experiences with vaccines, misinformation, concerns about vaccine side effects and political ideology. We combine our insights from this comprehensive review of global literature to offer an important and practical discussion about two strategies that have been used to improve vaccine uptake: (i) communication and education and (ii) vaccine rollout and logistics.
Keywords: COVID-19; SARS-CoV-2; pandemic planning; scoping review; vaccine hesitancy.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Similar articles
-
The Worrying Phenomenon of COVID-19 Vaccine Hesitancy and Its Negative Impact on Pandemic Control Efforts: Common Themes that Emerged in the Middle East and North Africa (MENA) Region.Adv Exp Med Biol. 2024;1457:299-322. doi: 10.1007/978-3-031-61939-7_17. Adv Exp Med Biol. 2024. PMID: 39283434 Review.
-
Interventions to increase COVID-19 vaccine uptake: a scoping review.Cochrane Database Syst Rev. 2022 Aug 3;8(8):CD015270. doi: 10.1002/14651858.CD015270. Cochrane Database Syst Rev. 2022. PMID: 35920693 Free PMC article.
-
What factors promote vaccine hesitancy or acceptance during pandemics? A systematic review and thematic analysis.Health Promot Int. 2022 Feb 17;37(1):daab105. doi: 10.1093/heapro/daab105. Health Promot Int. 2022. PMID: 34244738
-
COVID-19 vaccine hesitancy in Zambia: a glimpse at the possible challenges ahead for COVID-19 vaccination rollout in sub-Saharan Africa.Hum Vaccin Immunother. 2022 Dec 31;18(1):1-6. doi: 10.1080/21645515.2021.1948784. Epub 2021 Jul 6. Hum Vaccin Immunother. 2022. PMID: 34227914 Free PMC article.
-
COVID-19 vaccine hesitancy in Africa: a scoping review.Glob Health Res Policy. 2022 Jul 19;7(1):21. doi: 10.1186/s41256-022-00255-1. Glob Health Res Policy. 2022. PMID: 35850783 Free PMC article.
Cited by
-
Effectiveness of a Counseling Intervention to Increase Vaccination Uptake among Men Who Have Sex with Men during the Mpox Outbreak.Vaccines (Basel). 2024 Jul 8;12(7):751. doi: 10.3390/vaccines12070751. Vaccines (Basel). 2024. PMID: 39066389 Free PMC article.
-
Psychological and Social Aspects of Vaccination Hesitancy-Implications for Travel Medicine in the Aftermath of the COVID-19 Crisis: A Narrative Review.Medicina (Kaunas). 2023 Sep 28;59(10):1744. doi: 10.3390/medicina59101744. Medicina (Kaunas). 2023. PMID: 37893462 Free PMC article. Review.
-
Mental Health and Contraceptive Knowledge in High Schoolers: Comparing Remote and In-Person Learning during COVID-19.Medicina (Kaunas). 2023 Oct 22;59(10):1876. doi: 10.3390/medicina59101876. Medicina (Kaunas). 2023. PMID: 37893594 Free PMC article.
-
Previous Vaccination History and Psychological Factors as Significant Predictors of Willingness to Receive Mpox Vaccination and a Favorable Attitude towards Compulsory Vaccination.Vaccines (Basel). 2023 Apr 25;11(5):897. doi: 10.3390/vaccines11050897. Vaccines (Basel). 2023. PMID: 37243001 Free PMC article.
-
Determinants of Ukrainian Mothers' Intentions to Vaccinate Their Children in Poland: A Cross-Sectional Study.Vaccines (Basel). 2025 Mar 19;13(3):325. doi: 10.3390/vaccines13030325. Vaccines (Basel). 2025. PMID: 40266242 Free PMC article.
References
-
- Ahmad, M., Truong, J. and Majid, U. (2020) Let’s talk COVID-19 vaccine hesitancy. Longwoods. https://www.longwoods.com/content/26286//let-s-talk-covid-19-vaccine-hes... (last accessed 20 February 2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous