Brief Report: Chylothorax and Chylous Ascites During RET Tyrosine Kinase Inhibitor Therapy
- PMID: 35788405
- PMCID: PMC9427698
- DOI: 10.1016/j.jtho.2022.06.008
Brief Report: Chylothorax and Chylous Ascites During RET Tyrosine Kinase Inhibitor Therapy
Abstract
Introduction: Spontaneous chylous effusions are rare; however, they have been observed by independent investigators in patients treated with RET tyrosine kinase inhibitors (TKIs).
Methods: This multicenter, retrospective study evaluated the frequency of chylous effusions in patients treated with RET TKIs. Clinicopathologic features and management of patients with chylous effusions were evaluated.
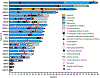
Results: A pan-cancer cohort of 7517 patients treated with one or more multikinase inhibitor or selective RET TKI and a selective TKI cohort of 96 patients treated with selpercatinib or pralsetinib were analyzed. Chylous effusions were most common with selpercatinib (7%), followed by agerafenib (4%), cabozantinib (0.3%), and lenvatinib (0.02%); none were observed with pralsetinib. Overall, 12 patients had chylothorax, five had chylous ascites, and five had both. Time from TKI initiation to diagnosis ranged from 0.5 to 50 months. Median fluid triglyceride level was lower in chylothoraces than in chylous ascites (397 mg/dL [interquartile range: 304-4000] versus 3786 mg/dL [interquartile range: 842-6596], p = 0.035). Malignant cells were present in 13% (3 of 22) of effusions. Chyle leak was not identified by lymphangiography. After initial drainage, 76% of patients with chylothorax and 80% with chylous ascites required additional interventions. Selpercatinib dose reduction and discontinuation rates in those with chylous effusions were 47% and 0%, respectively. Median time from diagnosis to disease progression was not reached (95% confidence interval: 14.5-undefined); median time from diagnosis to TKI discontinuation was 11.4 months (95% confidence interval: 8.2-14.9).
Conclusions: Chylous effusions can emerge during treatment with selected RET TKIs. Recognition of this side effect is key to prevent potential misattribution of worsening effusions to progressive malignancy.
Keywords: Chylothorax; Chylous ascites; Non–small cell lung cancer; RET tyrosine kinase inhibitor; Selpercatinib; Thyroid cancer.
Copyright © 2022 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Chylous Ascites as a Possible Rare Side Effect of Selpercatinib in RET-Positive NSCLC.J Thorac Oncol. 2023 Feb;18(2):e11-e13. doi: 10.1016/j.jtho.2022.10.012. J Thorac Oncol. 2023. PMID: 36682840 No abstract available.
-
Ascites During Selpercatinib Treatment: Need for a Multidisciplinary Approach.J Thorac Oncol. 2023 Feb;18(2):e9-e10. doi: 10.1016/j.jtho.2022.09.229. J Thorac Oncol. 2023. PMID: 36682848 No abstract available.
References
-
- Drilon A, Hu ZI, Lai GGY et al. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 2018; 15 (3): 150. - PubMed
-
- Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer 2014; 14 (3): 173–186. - PubMed
-
- Gainor JF, Curigliano G, Kim DW et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol 2021; 22 (7): 959–969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

