AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett's Esophagus: Expert Review
- PMID: 35788412
- PMCID: PMC10203866
- DOI: 10.1016/j.cgh.2022.06.003
AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett's Esophagus: Expert Review
Abstract
Description: The purpose of this best practice advice (BPA) article from the Clinical Practice Update Committee of the American Gastroenterological Association is to provide an update on advances and innovation regarding the screening and surveillance of Barrett's esophagus.
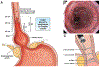
Methods: The BPA statements presented here were developed from expert review of existing literature combined with discussion and expert opinion to provide practical advice. Formal rating of the quality of evidence or strength of BPAs was not the intent of this clinical practice update. This expert review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee (CPUC) and the AGA Governing Board to provide timely guidance on a topic of high clinical importance to the AGA membership, and underwent internal peer review by the CPUC and external peer review through standard procedures of Clinical Gastroenterology and Hepatology. BEST PRACTICE ADVICE 1: Screening with standard upper endoscopy may be considered in individuals with at least 3 established risk factors for Barrett's esophagus (BE) and esophageal adenocarcinoma, including individuals who are male, non-Hispanic white, age >50 years, have a history of smoking, chronic gastroesophageal reflux disease, obesity, or a family history of BE or esophageal adenocarcinoma. BEST PRACTICE ADVICE 2: Nonendoscopic cell-collection devices may be considered as an option to screen for BE. BEST PRACTICE ADVICE 3: Screening and surveillance endoscopic examination should be performed using high-definition white light endoscopy and virtual chromoendoscopy, with endoscopists spending adequate time inspecting the Barrett's segment. BEST PRACTICE ADVICE 4: Screening and surveillance exams should define the extent of BE using a standardized grading system documenting the circumferential and maximal extent of the columnar lined esophagus (Prague classification) with a clear description of landmarks and the location and characteristics of visible lesions (nodularity, ulceration), when present. BEST PRACTICE ADVICE 5: Advanced imaging technologies such as endomicroscopy may be used as adjunctive techniques to identify dysplasia. BEST PRACTICE ADVICE 6: Sampling during screening and surveillance exams should be performed using the Seattle biopsy protocol (4-quadrant biopsies every 1-2 cm and target biopsies from any visible lesion). BEST PRACTICE ADVICE 7: Wide-area transepithelial sampling may be used as an adjunctive technique to sample the suspected or established Barrett's segment (in addition to the Seattle biopsy protocol). BEST PRACTICE ADVICE 8: Patients with erosive esophagitis should be biopsied when concern of dysplasia or malignancy exists. A repeat endoscopy should be performed after 8 weeks of twice a day proton pump inhibitor therapy. BEST PRACTICE ADVICE 9: Tissue systems pathology-based prediction assay may be utilized for risk stratification of patients with nondysplastic BE. BEST PRACTICE ADVICE 10: Risk stratification models may be utilized to selectively identify individuals at risk for Barrett's associated neoplasia. BEST PRACTICE ADVICE 11: Given the significant interobserver variability among pathologists, the diagnosis of BE-related neoplasia should be confirmed by an expert pathology review. BEST PRACTICE ADVICE 12: Patients with BE-related neoplasia should be referred to endoscopists with expertise in advanced imaging, resection, and ablation. BEST PRACTICE ADVICE 13: All patients with BE should be placed on at least daily proton pump inhibitor therapy. BEST PRACTICE ADVICE 14: Patients with nondysplastic BE should undergo surveillance endoscopy in 3 to 5 years. BEST PRACTICE ADVICE 15: In patients undergoing surveillance after endoscopic eradication therapy, random biopsies should be taken of the esophagogastric junction, gastric cardia, and the distal 2 cm of the neosquamous epithelium as well as from all visible lesions, independent of the length of the original BE segment.
Copyright © 2022 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors disclose the following. V. Raman Muthusamy reports consultant for Medtronic, Boston Scientific, research support from Boston Scientific; Advisory Board: Endogastric Solutions; honoraria from Torax Medical/Ethicon; and stock from Capsovision. Sachin Wani reports consultant for Exact Sciences advisory board for Cernostics; and research support from Lucid Diagnostics and CDx Diagnostics. Prakash Gyawali reports consultant for Medtronic. Srinadh Komanduri reports consultant for Medtronic, Boston Scientific, Castle Biosciences, and Johnson & Johnson.
Figures

References
-
- ASGE Standards of Practice Committee, Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc 2019;90:335–359.e2. - PubMed
-
- Qumseya BJ, Bukannan A, Gendy S, et al. Systematic review and meta-analysis of prevalence and risk factors for Barrett’s esophagus. Gastrointest Endosc 2019;90:707–717.e1. - PubMed
-
- Sawas T, Zamani SA, Killcoyne S, et al. Limitations of heartburn and other societies’ criteria in Barrett’s screening for detecting de novo esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2021. (Online ahead of print). - PubMed

